Abstract
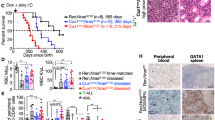
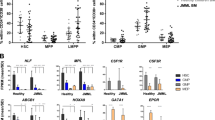
Oncogenic NRAS and KRAS mutations are prevalent in human juvenile and chronic myelomonocytic leukemia (JMML/CMML). However, additional genetic mutations cooperating with oncogenic RAS in JMML/ CMML progression and/or their transformation to acute myeloid leukemia (AML) remain largely unknown. Here we tested the potential genetic interaction of DNMT3A mutations and oncogenic RAS mutations in leukemogenesis. We found that Dnmt3a−/− induces multiple hematopoietic phenotypes after a prolonged latency, including T-cell expansion in the peripheral blood, stress erythropoiesis in the spleen and myeloid malignancies in the liver. Dnmt3a−/− significantly promoted JMML/CMML progression and shortened the survival of KrasG12D/+ mice in a cell-autonomous manner. Similarly, downregulating Dnmt3a also promoted myeloid malignancies in NrasG12D/+ mice. Further studies show that Dnmt3a deficiency rescues KrasG12D/+-mediated depletion of hematopoietic stem cells and increases self-renewal of KrasG12D/+ myeloid progenitors (MPs). Moreover, ~33% of animals developed an AML-like disease, which is driven by KrasG12D/+; Dnmt3a−/− MPs. Consistent with our result, COSMIC database mining demonstrates that the combination of oncogenic RAS and DNMT3A mutations exclusively occurred in patients with JMML, CMML or AML. Our results suggest that DNMT3A mutations and oncogenic RAS cooperate to regulate hematopoietic stem and progenitor cells and promote myeloid malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ward AF, Braun BS, Shannon KM . Targeting oncogenic Ras signaling in hematologic malignancies. Blood 2012; 120: 3397–3406.
Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci USA 2004; 101: 597–602.
Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest 2004; 113: 528–538.
Zhang J, Wang J, Liu Y, Sidik H, Young KH, Lodish HF et al. Oncogenic Kras-induced leukemogeneis: hematopoietic stem cells as the initial target and lineage-specific progenitors as the potential targets for final leukemic transformation. Blood 2009; 113: 1304–1314.
Du J, Liu Y, Meline B, Kong G, Tan LX, Lo JC et al. Loss of CD44 attenuates aberrant GM-CSF signaling in Kras G12D hematopoietic progenitor/precursor cells and prolongs the survival of diseased animals. Leukemia 2013; 27: 754–757.
Wang JY, Liu YG, Li ZY, Du J, Ryu MJ, Taylor PR et al. Endogenous oncogenic Nras mutation leads to aberrant GM-CSF signaling in granulocytic/monocytic precursors in a murine model of chronic myelomonocytic leukemia. Blood 2010; 116: 5991–6002.
Li Q, Haigis KM, McDaniel A, Harding-Theobald E, Kogan SC, Akagi K et al. Hematopoiesis and leukemogenesis in mice expressing oncogenic NrasG12D from the endogenous locus. Blood 2011; 117: 2022–2032.
Wang JY, Liu YG, Li ZY, Wang ZD, Tan LX, Ryu MJ et al. Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a dose- and cell type-dependent manner. Blood 2011; 118: 368–379.
Shih AH, Abdel-Wahab O, Patel JP, Levine RL . The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer 2012; 12: 599–612.
Dunbar AJ, Gondek LP, O'Keefe CL, Makishima H, Rataul MS, Szpurka H et al. 250 K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res 2008; 68: 10349–10357.
Kohlmann A, Grossmann V, Klein HU, Schindela S, Weiss T, Kazak B et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol 2010; 28: 3858–3865.
Kato M, Yasui N, Seki M, Kishimoto H, Sato-Otsubo A, Hasegawa D et al. Aggressive transformation of juvenile myelomonocytic leukemia associated with duplication of oncogenic KRAS due to acquired uniparental disomy. J Pediatr 2013; 162: 1285–1288.
Chang YI, Damnernsawad A, Allen LK, Yang D, Ranheim EA, Young KH et al. Evaluation of allelic strength of human TET2 mutations and cooperation between Tet2 knockdown and oncogenic Nras mutation. Br J Haematol 2014; 166: 461–465.
Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010; 363: 2424–2433.
Li KK, Luo LF, Shen Y, Xu J, Chen Z, Chen SJ . DNA methyltransferases in hematologic malignancies. Semin Hematol 2013; 50: 48–60.
Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell 2014; 25: 442–454.
Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T . A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood 2013; 122: 4086–4089.
Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 2012; 44: 23–31.
Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood 2014; 125: 629–638.
Gao Q, Steine EJ, Barrasa MI, Hockemeyer D, Pawlak M, Fu D et al. Deletion of the de novo DNA methyltransferase Dnmt3a promotes lung tumor progression. Proc Natl Acad Sci USA 2011; 108: 18061–18066.
Network TCGAR. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074.
Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R . Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev Dyn 2007; 236: 1663–1676.
Kong G, Wunderlich M, Yang D, Ranheim EA, Young KH, Wang J et al. Combined MEK and JAK inhibition abrogates murine myeloproliferative neoplasm. J Clin Invest 2014; 124: 2762–2773.
Kuhn R, Schwenk F, Aguet M, Rajewsky K . Inducible gene targeting in mice. Science 1995; 269: 1427–1429.
Peters SL, Hlady RA, Opavsky J, Klinkebiel D, Pirruccello SJ, Talmon GA et al. Tumor suppressor functions of Dnmt3a and Dnmt3b in the prevention of malignant mouse lymphopoiesis. Leukemia 2014; 28: 1138–1142.
Sabnis AJ, Cheung LS, Dail M, Kang HC, Santaguida M, Hermiston ML et al. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol 2009; 7: e59.
Celik H, Mallaney C, Kothari A, Ostrander EL, Eultgen E, Martens A et al. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood 2014; 125: 619–628.
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014; 506: 328–333.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014; 20: 1472–1478.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488–2498.
Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014; 371: 2477–2487.
Wang J, Kong G, Liu Y, Du J, Chang Y-I, Zhang X et al. Nras G12D/+ promotes leukemogenesis by aberrantly regulating haematopoietic stem cell functions. Blood 2013; 121: 5203–5207.
Parikh C, Subrahmanyam R, Ren R . Oncogenic NRAS rapidly and efficiently induces CMML- and AML-like diseases in mice. Blood 2006; 108: 2349–2357.
Acknowledgements
We are grateful to Dr Qiang Chang for providing the Dnmt3a conditional knockout mice. We thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services to complete this research. This work was supported by R01 grants R01CA152108 and R01HL113066, and a Scholar Award from the Leukemia & Lymphoma Society to JZ. Y-IC was supported by the Ministry of Science and Technology (MOST 103-2320-B-010-047) and a grant from Ministry of Education, Aim for the Top University Plan. This work was also supported in part by NIH/NCI P30 CA014520–UW Comprehensive Cancer Center Support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Chang, YI., You, X., Kong, G. et al. Loss of Dnmt3a and endogenous KrasG12D/+ cooperate to regulate hematopoietic stem and progenitor cell functions in leukemogenesis. Leukemia 29, 1847–1856 (2015). https://doi.org/10.1038/leu.2015.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.85
This article is cited by
-
Neoantigen-specific TCR-T cell-based immunotherapy for acute myeloid leukemia
Experimental Hematology & Oncology (2022)
-
DNA methylation: a saga of genome maintenance in hematological perspective
Human Cell (2022)
-
Mutations in chronic myelomonocytic leukemia and their prognostic relevance
Clinical and Translational Oncology (2021)
-
Pediatric Neoplasms Presenting with Monocytosis
Current Hematologic Malignancy Reports (2021)
-
Functional and epigenetic phenotypes of humans and mice with DNMT3A Overgrowth Syndrome
Nature Communications (2021)