Abstract
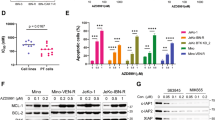
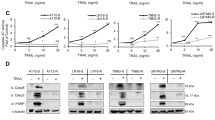
The development of resistance to therapy is unavoidable in the history of multiple myeloma patients. Therefore, the study of its characteristics and mechanisms is critical in the search for novel therapeutic approaches to overcome it. This effort is hampered by the absence of appropriate preclinical models, especially those mimicking acquired resistance. Here we present an in vivo model of acquired resistance based on the continuous treatment of mice bearing subcutaneous MM1S plasmacytomas. Xenografts acquired resistance to two generations of immunomodulatory drugs (IMiDs; lenalidomide and pomalidomide) in combination with dexamethasone, that was reversible after a wash-out period. Furthermore, lenalidomide–dexamethasone (LD) or pomalidomide–dexamethasone (PD) did not display cross-resistance, which could be due to the differential requirements of the key target Cereblon and its substrates Aiolos and Ikaros observed in cells resistant to each combination. Differential gene expression profiles of LD and PD could also explain the absence of cross-resistance. Onset of resistance to both combinations was accompanied by upregulation of the mitogen-activated protein kinase/extracellular signal–regulated kinase (ERK) kinase (MEK)/ERK pathway and addition of selumetinib, a small-molecule MEK inhibitor, could resensitize resistant cells. Our results provide insights into the mechanisms of acquired resistance to LD and PD combinations and offer possible therapeutic approaches to addressing IMiD resistance in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Palumbo A, Anderson K . Multiple myeloma. N Engl J Med. 2011; 364: 1046–1060.
Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia 2014; 28: 525–542.
Mitsiades CS, Hideshima T, Chauhan D, McMillin DW, Klippel S, Laubach JP et al. Emerging treatments for multiple myeloma: beyond immunomodulatory drugs and bortezomib. Semin Hematol. 2009; 46: 166–175.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y et al. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327: 1345–1350.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014; 343: 305–309.
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014; 164: 811–821.
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343: 301–305.
Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J 1997; 16: 2004–2013.
Licht JD, Shortt J, Johnstone R . From anecdote to targeted therapy: the curious case of thalidomide in multiple myeloma. Cancer Cell 2014; 25: 9–11.
Gandhi AK, Mendy D, Waldman M, Chen G, Rychak E, Miller K et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol 2014; 164: 233–244.
Greenberg AJ, Walters DK, Kumar SK, Vincent Rajkumar S, Jelinek DF . Responsiveness of cytogenetically discrete human myeloma cell lines to lenalidomide: lack of correlation with cereblon and interferon regulatory factor 4 expression levels. Eur J Haematol 2013; 91: 504–513.
Heintel D, Rocci A, Ludwig H, Bolomsky A, Caltagirone S, Schreder M et al. High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Br J Haematol 2013; 161: 695–700.
Schuster SR, Kortuem KM, Zhu YX, Braggio E, Shi C-X, Bruins L et al. Cereblon expression predicts response, progression free and overall survival after pomalidomide and dexamethasone therapy in multiple myeloma. ASH Annu Meeting Abstr 2012; 120: 194.
Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011; 118: 4771–4779.
Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012; 26: 2326–2335.
Maiga S, Gomez-Bougie P, Bonnaud S, Gratas C, Moreau P, Le Gouill S et al. Paradoxical effect of lenalidomide on cytokine/growth factor profiles in multiple myeloma. Br J Cancer 2013; 108: 1801–1806.
Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM et al. Evidence of a role for activation of Wnt/{beta}-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem 2010; 286: 11009–11020.
Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood 2012; 120: 376–385.
Ocio EM, Vilanova D, Atadja P, Maiso P, Crusoe E, Fernandez-Lazaro D et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica 2010; 95: 794–803.
Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res 2006; 66: 5781–5789.
Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R . Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013; 160: 487–502.
Gutierrez NC, Sarasquete ME, Misiewicz-Krzeminska I, Delgado M, De Las Rivas J, Ticona FV et al. Deregulation of microRNA expression in the different genetic subtypes of multiple myeloma and correlation with gene expression profiling. Leukemia 2010; 24: 629–637.
Lopez-Corral L, Sarasquete ME, Bea S, Garcia-Sanz R, Mateos MV, Corchete LA et al. SNP-based mapping arrays reveal high genomic complexity in monoclonal gammopathies, from MGUS to myeloma status. Leukemia 2012; 26: 2521–2529.
Zhang J, Feuk L, Duggan GE, Khaja R, Scherer SW . Development of bioinformatics resources for display and analysis of copy number and other structural variants in the human genome. Cytogenet Genome Res 2006; 115: 205–214.
Thorvaldsdottir H, Robinson JT, Mesirov JP . Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinformatics 2013; 14: 178–192.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G et al. Integrative genomics viewer. Nat Biotechnol 2011; 29: 24–26.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007; 357: 2123–2132.
Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia 2009; 23: 2147–2152.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 2007; 357: 2133–2142.
San-Miguel JF, Weisel KC, Moreau P, Lacy M, Song KW, Delforge M et al. MM-003: A phase III, multicenter, randomized, open-label study of pomalidomide (POM) plus low-dose dexamethasone (LoDEX) versus high-dose dexamethasone (HiDEX) in relapsed/refractory multiple myeloma (RRMM). ASCO Meeting Abstr 2013; 31 (Suppl): 8510.
Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012; 120: 2817–2825.
Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol 2012; 158: 739–748.
Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia 2010; 24: 1934–1939.
Lacy MQ, Kumar SK, LaPlant BR, Laumann K, Gertz MA, Hayman SR et al. Pomalidomide plus low-dose dexamethasone (Pom/Dex) in relapsed myeloma: long term follow up and factors predicing outcome in 345 patients. ASH Annu Meeting Abstr 2012; 120: 201.
Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood 2011; 118: 2970–2975.
Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood 2013; 121: 1968–1975.
San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14: 1055–1066.
Thakurta A, Gandhi AK, Waldman MF, Bjorklund C, Ning Y, Mendy D et al. Absence of mutations in cereblon (CRBN) and DNA damage-binding protein 1 (DDB1) genes and significance for IMiD therapy. Leukemia 2013; 28: 1129–1131.
Walker BA, Wardell CP, Melchor L, Brioli A, Johnson DC, Kaiser MF et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 2013; 28: 384–390.
Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood 2012; 120: 1060–1066.
Holkova B, Badros AZ, Geller R, Voorhees PM, Zingone A, Korde N et al. A phase II study of the MEK 1/2 inhibitor AZD6244 (Selumetinib, ARRY-142866) in relapsed or refractory multiple myeloma. ASH Annu Meeting Abstr 2011; 118: 2931.
Acknowledgements
The present work was funded by a grant from the Spanish Ministry of Science and Technology (FIS PI 11/01465); the Asociación Española Contra el Cáncer (AECC) grant (GCB120981SAN); and Cooperative Research Thematic Networks (RTICC) grant numbers RD06/0020/0006, RD12/0036/0058 and RD12/0036/0003. EDR has also been awarded with an AECC grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EMO received research funding from Celgene. JFSM discloses consultancy and honoraria from Celgene. SC, MW and CCB are employees of Celgene Corporation, Summit, NJ, USA. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Ocio, E., Fernández-Lázaro, D., San-Segundo, L. et al. In vivo murine model of acquired resistance in myeloma reveals differential mechanisms for lenalidomide and pomalidomide in combination with dexamethasone. Leukemia 29, 705–714 (2015). https://doi.org/10.1038/leu.2014.238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.238
This article is cited by
-
Efficacy and safety of pomalidomide, bortezomib, and dexamethasone combination chemotherapy for newly diagnosed multiple myeloma: POMACE Phase II Study
Blood Cancer Journal (2023)
-
Pomalidomide, dexamethasone, and daratumumab in Japanese patients with relapsed or refractory multiple myeloma after lenalidomide-based treatment
International Journal of Hematology (2022)
-
Key regulators of sensitivity to immunomodulatory drugs in cancer treatment
Biomarker Research (2021)
-
Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): outcomes by prior treatment at first relapse
Leukemia (2021)
-
Combined targeting of MEK and the glucocorticoid receptor for the treatment of RAS-mutant multiple myeloma
BMC Cancer (2020)