Abstract
Chimeric antigen receptor (CAR)-redirected cellular therapy is an attractive modality for cancer treatment. We hypothesized that allogeneic CAR-engineered CD45RA-negative T cells can control cancer and infection without the risk of graft-versus-host disease (GVHD). We used CD19+ MLL-rearranged leukemia as prototype because it is an aggressive and generally drug-resistant malignancy. CD45RA– cells that were transduced with anti-CD19 CAR containing 4-1BB and CD3ζ signaling domains effectively lysed MLL-rearranged leukemia cell lines and primary blasts in vitro. In a disseminated leukemia mouse model, CAR+CD45RA– cells significantly reduced leukemia burdens and prolonged overall survival without GVHD. CAR+ cells were sustainable in blood, and all the treated mice remained leukemia-free even after they were re-challenged with leukemia cells. Despite the transduction process, CD45RA– cells retained recall activity both in vitro and in vivo against human pathogens commonly found in cancer patients. In comparison with CD45RA+ cells, CD45RA– cells showed less allogeneic activity in mixed leukocyte reactions and in mouse models. Thus, the use of CAR+CD45RA– cells can separate GVHD from graft-versus-malignancy effect and infection control. These cells should also be useful in nontransplant settings and may be administered as off-the-shelf third-party cells.
Similar content being viewed by others

Introduction
More than 10 000 hematopoietic cell transplantations (HCTs) are performed worldwide annually for the treatment of leukemia.1 Although the outcomes have steadily improved over the years, leukemia relapse after HCT remains the most common cause of failure.2 Treatment options are limited for these patients because leukemia is usually resistant to chemotherapy and donor lymphocyte infusion, and the patients often have comorbid conditions such as organ dysfunction or concurrent infections. Moreover, salvage chemotherapy and donor lymphocyte infusion have many side effects, including leukopenia and graft-versus-host disease (GVHD), that may further increase the risk of infections. Thus, novel therapies with distinct mechanisms of action and no overlapping toxicities are needed.
We hypothesized that CD45RA– T cells expressing chimeric antigen receptor (CAR) against a leukemia-associated antigen are ideal effectors for leukemia control. CD45RA and CD45RO are isoforms of CD45 (also called leukocyte common antigen, because it is found on all cells of hematopoietic origin except erythrocytes). In T lymphocytes, CD45RA is expressed on naive cells, whereas CD45RO is expressed on memory cells.3 CD45RA+ naive T cells have a high potential for alloreactivity against recipient-specific antigens after adoptive transfer, resulting in clinical GVHD.4, 5 In contrast, CD45RO+ T cells exert a memory response to prior pathogens or vaccines, thus providing the recipient immediate infection immunity. Furthermore, murine studies have shown that memory CD4 T cells can mediate a graft-versus-leukemia effect without inducing GVHD.6, 7
To improve their specificity and potency against leukemia, CD45RA– cells can be genetically modified to express a CAR specific for a leukemia lineage-associated antigen, such as CD19/CD20/CD22 in B-lineage and CD33/CD123 in myeloid-lineage hematologic malignancies.8, 9, 10, 11 CARs that are being actively investigated are typically recombinant artificial receptors that contain a single-chain variable fragment of an antibody coupled to the signaling domains of activation molecules.12, 13, 14 There are several generations of CARs with modifications on the signaling motifs.15 CARs can be introduced into effector cells by using gammaretroviral,16 lentiviral,17 mRNA18, 19 or Sleeping Beauty transposon/ transposase systems.20 CAR-modified cells are currently undergoing clinical trials for various cancers, including hematologic malignancies and solid tumors.21 Current optimization strategies include single-chain variable fragment selection,22 epitope binding,8 vector construction,15 cell transduction23 and clinical-scale production.24
Here we provide evidence to support our hypothesis that CAR-modified CD45RA– T cells are effective and safe for leukemia control. We used MLL-rearranged leukemia as a prototype because it is associated with a poor prognosis and frequent relapse after HCT.25, 26 We found that anti-CD19 CAR+CD45RA– cells not only could lyse CD19+ natural killer (NK) cell-resistant MLL-rearranged leukemia cells, but also have memory responses to common viral antigens. Importantly, the CAR+CD45RA– T cells have no GVHD activity and can prolong survival in mouse models of refractory leukemia and those rechallenged with leukemia.
Materials and methods
Cell isolation and vector production
Peripheral blood mononuclear cells (PBMCs) were collected from healthy donors with approval from the St Jude Children’s Research Hospital institutional review board and written informed consent from donors. For the depletion of CD45RA+ cells, PBMCs were labeled with CD45RA microbeads (Miltenyi Biotec, Auburn, CA, USA) and depleted using autoMACS or CliniMACS (both from Miltenyi Biotec) following the manufacturer’s instructions. The purities of the CD45RA-negative fractions were always >99%, as determined by flow cytometry. The HL20i4r-MND-antiCD19bbz and HL20i4r-EF1α-antiCD19bbz lentiviral vectors were derived from the clinical vector CL20i4r-EF1α-hgcOPT27 but expressed an anti-CD19 CAR designed by Imai et al.28 and tested clinically by Porter et al.17 It contained the single-chain variable fragment of anti-CD19 antibody, the hinge region of CD8α and the signaling domains of 4-1BB and CD3-ζ. The cassette was driven by either MND or elongation factor 1α (EF1α) promoters and insulated from surrounding chromatin with the 400 bp chicken HS4 element.29 Viral supernatant was produced by transient transfection of HEK293T cells with the vector genome plasmid and lentiviral packaging helper plasmids pCAGG-HIVgpco, pCAGG-VSVG and pCAG4-RTR2. The supernatant was concentrated by ultra-centrifugation and titrated on HeLa cells by serial dilution followed by quantitative PCR to determine vector genome copy number.
CAR transduction
Our transduction protocol for both small-scale and clinical-scale experiments required only 5 days of cell processing. On day −5, cells were primed at 5 × 106 /ml overnight in a culture cocktail consisting of XVIVO-15 (Lonza, Walkersville, MD, USA), 250 U/ml IL-2 (Novartis Pharmaceuticals, Emeryville, CA, USA), 10 ng/ml OKT3 (BioLegend, San Diego, CA, USA) and 10 ng/ml anti-CD28 antibody (BioLegend). On Days −4 and −3, transduction was performed daily at 1 × 106 cells/ml on RetroNectin-coated plates at 10 μg/cm2 according to the manufacturer’s instructions (Takara Bio, Clontech Laboratories, Mountain View, CA, USA). For large-scale transduction, VueLife culture bags were used (American Fluoroseal Corporation, Gaithersburg, MD, USA). Cells were harvested on day 0 for experiments.
Flow cytometry
Cell phenotypes were analyzed by flow cytometry. The following antibody clones were used: anti-CD3 (SK7, UCHT1), anti-CD56 (MY31, N901), anti-CD14 (MphiP9), anti-CD45RA (L48), anti-CD45RO (UCHL1), anti-CCR7 (150503), anti-CD4 (SK3) and anti-CD8 (T8). For the detection of CAR, biotinylated recombinant protein L (Thermo Scientific, Pittsburgh, PA, USA) was used together with R-phycoerythrin fluorescent-labeled secondary anti-biotin antibody (BioLegend). Analyses were performed with BD LSRFortessa (BD Biosciences, San Jose, CA, USA) and FlowJo 10.0 software (Tree Star, Ashland, OR, USA).
Leukemia cell lines and primary blasts
The CD19+ MLL-rearranged cell-lines RS4;11 (American Type Culture Collection, Manassas, VA, USA) and SEM (DSMZ, Braunschweig, Germany), together with CD19– MV4-11 control (American Type Culture Collection), were cultured and authenticated regularly as previously reported.26 Fresh primary MLL-rearranged blasts were obtained from a patient with t(4;11) diagnosed at 2 months of age under informed consent and institutional review board approval. The lymphoblasts were CD19+, CD33+ and CD7dim/+ and were established as a cell line (named SJ4-11) after serial passage subcutaneously in the rear flanks of NOD-SCID IL-2γc−/− (NOG) mice. All animal experiments were approved by the St Jude animal care and use committee.
Cytotoxicity assay
Cytotoxic activity was measured by using the DELFIA BATDA reagent (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) and a flow-based assay following the manufacturer’s instructions. BATDA-labeled MLL cells were used as target cells at an effector/target (E/T) ratio of 5:1 for 2 h at 37 °C. The fluorescence signals were measured using a Wallac Victor 2 Counter Plate Reader (PerkinElmer Life and Analytical Sciences). For the flow-based cytotoxicity assay, fluorescent dye Calcein-AM (Sigma, St Louis, MO, USA) was used to label target cells.
Antigen recall experiments
The memory responses of CD45RA– and CD45RA+ fractions were examined in vitro using a DELFIA cell proliferation kit (PerkinElmer Life and Analytical Sciences). Briefly, 1 × 105 cells were seeded into 96-well flat-bottom plates and challenged with viral lysates including human cytomegalovirus (CMV), Epstein–Barr virus and herpes simplex virus (all from Advanced Technologies Inc., Columbia, MD, USA) and tetanus toxoid (Sanofi Pasteur, Swiftwater, PA, USA) for 5 days. Proliferation was measured by the incorporation of 5-bromo-2'-deoxyuridine (BrdU) as detected in the proliferating cells. The BrdU counts were read using a Wallac Victor 2 Counter Plate Reader (PerkinElmer Life and Analytical Sciences). Phytohemagglutinin (Sigma) was used as a positive control for cell proliferation.
For in vivo antigen recall experiments, blood samples from CMV-seropositive, asymptomatic healthy donors were screened for HLA-A*0201. CD45RA– and CD45RA+ fractions were isolated from CMV+ HLA-A*0201+ PBMCs and injected intraperitoneally at 10 × 106 per NOG mouse. After 1 week, the mice were either challenged with phosphate-buffered saline as mock control or with 5 × 106 monocyte-derived dendritic cells (derived from the same donor) pulsed with human CMV viral lysate. Blood samples were drawn on day 7 to determine the cell counts of human CD45+ cells. On day 14, the mice were given intraperitoneal injections of BrdU (Sigma). They were killed using carbon dioxide the next day. Splenocytes were analyzed for proliferating cells using a BD FastImmune-BrdU Flow kit (BD Biosciences). The percentage of proliferating cells was gated based on human CD45 and BrdU. Percentages of CMV-tetramer+ cells were measured from the proliferating BrdU+ populations using HLA-A*0201-CMVpp65 tetramer-PE (iTag, Beckman Coulter, Pasadena, CA, USA).
Allogeneic mixed leukocyte reaction (MLR)
To compare the alloreactivity of CD45RA– and CD45RA+ cells, MLRs were performed with responder cells seeded into 96-well flat-bottom plates at 1 × 105 cells per well in triplicate. Unrelated third-party stimulator PBMCs were γ-irradiated at 3000 rad and mixed in a responder/stimulator ratio of 10:1. Wells containing responders or stimulators alone were used to set the background signals. Cell proliferation was measured by the incorporation of BrdU using a DELFIA cell proliferation kit (PerkinElmer Life and Analytical Sciences) according to the manufacturer’s instructions. Briefly, BrdU was added to the medium in each well on day 5. On the next day, the incorporated BrdU was stained by anti-BrdU antibody, developed and counted using a Wallac Victor 2 Counter Plate Reader (PerkinElmer Life and Analytical Sciences). In addition, the production of interferon-γ in the MLR supernatant was measured using enzyme-linked immunosorbent assay.
In vivo experiments
NOG mice 8–12 weeks old were used as a preclinical model to determine the efficacy and toxicity of the CD45RA-negative and -positive CAR cells. CD19+ SEM cells were transduced with gammaretroviral vector containing MSCV-luciferase-IRES-YFP (Vector Laboratory, St Jude, Memphis, TN, USA) as previously described.26 The YFP+ cells were used as the target. Mice were γ-irradiated at a dose of 100 cGy at 1 day before 1 × 106 SEM cells were intravenously injected. On the next day or on day 18, 10 × 106 CAR-transduced cells were intravenously injected. Human IL-2 (100 U/ml, Novartis Pharmaceuticals) was subcutaneously injected starting a day after injection of effector cells and continued every other day for 3 weeks. Disease progression was regularly monitored by bioluminescence imaging (Xenogen, PerkinElmer Life and Analytical Sciences), and survival data were recorded. The mice were killed when they displayed signs of significant leukemia symptoms, including paralysis or >20% weight loss. The experiments were terminated in surviving mice on day 90 after the injection of SEM cells or after the second leukemia challenge. All the sick or dead mice underwent autopsy, and their peritoneal fluids, blood and spleen were collected and immunophenotyped to determine the presence of human cells, including CAR+ cells and leukemia cells. The kinetics and persistence of CAR+ cells in vivo were documented by flow cytometric analyses of serial blood samples.
Statistical analysis
Statistical significance between two groups was calculated using Student’s paired t-test. In cases with more than two groups, one-way analysis of variance was used. For the survival analysis, Kaplan–Meier curves were constructed and compared by log-rank test. The nominal significance level was set at 0.05.
Results
CD45RA-depleted cell phenotype and CAR transduction
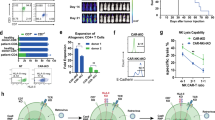
We analyzed the phenotypes of the negative and positive fractions after CD45RA depletion. In the negative fraction, there were few CD56+ NK cells, with greater enrichment of CD4+ T cells than of CD8+ T cells (Figure 1a). More than 99% of the cells were CD45RO+ (Figure 1b) and included both CCR7– effector memory and CCR7+ central memory T cells. In contrast, the positive fraction contained a large proportion of CD45RA+CCR7+ naive T cells with high potential for alloreactivity.
CD45RA-depleted cells are mainly CD3+CD45RO+ memory T cells and express a high level of chimeric antigen receptor after lentiviral transduction. (a) Phenotypic analysis of CD45RA-negative and -positive fractions. The expressions of CD3, CD56, CD4, CD8, CD45RO, CD45RA and CCR7 were determined and compared among PBMCs and the CD45RA– and CD45RA+ populations. (b) Percentage of CD45RA+ or CD45RO+ cells and yield after cell depletion. The median yield for CD45RA– cells was 58.6%. (c) Schematic presentation of lentiviral vector expressing anti-CD19 CAR used in this study. Ψ, ‘Psi’ packaging signal; cpPT, central polypurine tract; LTR+Ins., insulated long terminal repeat; MND: MND promoter; RRE: Rev-responsive element. Components of the anti-CD19 CAR are indicated below the figure. (d) Flow cytometric analysis of CAR expression on CD45RA– cells after lentiviral transduction at an MOI of 2.
Preliminary experiments in the CD45RA– cells corroborated earlier published work in mice showing enhanced expression of transgenes by the MND promoter relative to EF1α.30 The MND promoter showed no evidence of genotoxicity in a clinical trial for adrenoleukodystrophy,31 but was nonetheless inserted between chromatin insulators for added safety and enhanced reliability of gene expression. With the MND promoter (Figure 1c), the average CAR expression on CD45RA– cells was fivefold higher than that driven by EF1α, as judged by percentage of positive cells (data not shown). At a multiplicity of infection (MOI) of only 2, CD45RA– cells robustly expressed CAR with a median of 48.9% (Figure 1d). Thereafter, we used MND-driven CAR for the entire study.
Lysis of CD19+ MLL cells
To test the functions of the CAR+CD45RA– cells, we used three MLL-rearranged cell lines as target cells. The expression of CD19 on SEM was the highest, whereas MV4-11 cells did not have detectable CD19 (Figure 2a). CAR+CD45RA– cells could lyse both RS4;11 and SEM cells in a dose-dependent manner, but not MV4-11 cells. An average of 25% and 47% of RS4;11 and SEM cells, respectively, were lysed within 2 h at an E/T ratio of 20:1 (Figure 2b). Increasing the incubation time to 4 h increased the cytotoxicity against SEM to 62% at the same ratio (Figure 2c).
CAR+CD45RA– cells lysed CD19+ MLL-rearranged leukemia cells and primary blasts in a dose- and time-dependent manner. (a) Expression intensity of CD19 on RS4;11, SEM and MV4-11. Higher expression was observed on SEM (blue) than on RS4;11 (red), and expression was negligible on MV4-11 (green). (b) Cytotoxicity of CAR+CD45RA– cells on RS4;11, SEM and MV4-11 at different E/T ratios. (c) Temporal difference in cytotoxicity of CAR+CD45RA– cells on SEM cell line at different E/T ratios. The effector and targets were incubated for 2 or 4 h. (d) NK-resistant primary blasts were susceptible to CAR+CD45RA– cells. Cytotoxicity of NK and CAR+CD45RA– cells was assessed at different E/T ratios. RS4;11 and K562 were used as the negative and positive controls for the NK cells, respectively. Primary blasts (SJ4-11) were tested with NK and CAR+CD45RA– cells for comparison. Data are mean±s.e.m. from five independent experiments.
We then extended our investigation to include fresh primary MLL-rearranged leukemia blasts (SJ4-11). The cells were tested for sensitivity to NK cells and to CAR+CD45RA– cells that originated from the same healthy donor. The SJ4-11 blasts were resistant to NK lysis (less than 10% at all E/T ratios, similar to that of NK-resistant cell line RS4;11 and much lower than that of NK-sensitive K562; Figure 2d). However, the blasts were susceptible to CAR+CD45RA– cells.
Memory response against pathogens by CD45RA– cells
Because CD45RA– T cells had a memory phenotype, we hypothesized that they would have a better recall response than CD45RA+ cells to common pathogens and vaccines. As shown in Figure 3a, more cell proliferation was observed in the CD45RA– cells than in CD45RA+ cells consistently in all paired samples for human CMV, Epstein–Barr virus, herpes simplex virus and tetanus toxoid, whereas the nonspecific response to phytohemagglutinin mitogen was similar in the two cell fractions.
CD45RA– cells exert memory response against common pathogens without alloreactivity. (a) Cell proliferation responses of CD45RA– and CD45RA+ cells against human CMV, Epstein–Barr virus (EBV), herpes simplex virus (HSV) and tetanus toxoid (TT). The cells were stimulated with viral lysates or toxoid and incubated for 5 days. Cell proliferation was measured by the incorporation of BrdU. Phytohemagglutinin (PHA) was used as positive control. (b) Allogeneic MLR with CD45RA– or CD45RA+ cells. CD45RA– cells and CD45RA+ cells were used as the responders, and γ-irradiated third-party PBMCs were used as stimulators at a responder/stimulator ratio of 10:1. Data are mean±s.e.m. from three independent experiments. (c) Interferon-γ (IFN-γ) production from allogeneic MLRs of CD45RA– and CD45RA+ cells. The detection range was from 1 to 1000 pg/ml. Data are mean±s.e.m. from three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
CD45RA– cells had minimal alloreactivity in vitro
To determine the alloreactivity of the two cell fractions, CD45RA– and CD45RA+ cells (responders) were co-cultured with the same third-party irradiated PBMCs (stimulators). Compared with CD45RA– responders, CD45RA+ cells proliferated significantly much more in the MLR assays (Figure 3b). Furthermore, only the CD45RA+ MLRs produced high amounts of interferon-γ in the culture supernatant (Figure 3c).
Antileukemia activity of CAR+CD45RA– cells in vivo
A leukemia mouse model was used to evaluate the efficacy and safety of the CAR+CD45RA– cells. NOG mice were given intravenous injections of SEM cells that were transduced with luciferase and yellow fluorescent protein (YFP). CAR+CD45RA– cells were then adoptively transferred the next day at an E/T ratio of 10:1. The bioluminescence signals of the groups injected with SEM alone or with SEM plus CD45RA– untransduced cells increased over time. In contrast, the group that received CAR+CD45RA– cells at an MOI of 2 (CD45RA–MOI2) had no detectable signals until the end of the study on day 90 (Figure 4a). The difference in bioluminescence on day 14 among the three groups was statistically significant, both ventrally and dorsally (Figure 4b). All mice in the SEM-alone group and in the CD45RA–-untransduced group died before day 45, whereas 80% in the CD45RA– MOI2 group remained alive on day 90 (Figure 4c, P<0.01 compared with SEM alone or SEM plus CD45RA– untransduced). For the two deaths found on day 41 and day 62, no SEM cells were found in the blood, spleen or bone marrow samples. There was no significant weight loss or signs of toxicity before death.
During these in vivo experiments, we tracked the leukemia burden along with the number of CAR+ effector cells in the blood every week by flow cytometry using antibodies against human/mouse CD45, CD19 and YFP (Figure 4d). The leukemia cells were human CD45+CD19+YFP+ and the CAR+ cells were human CD45+CD19– YFP–. Of the 10 treated mice, CAR+ cells peaked from day 21 to day 31 and then contracted thereafter (Figure 4e, left panel). The number of leukemia cells increased rapidly in the mice of the SEM-alone group and SEM plus CD45RA– untransduced group, but not in the CD45RA–MOI2 group (Figure 4e, right panel).
Refractory and relapse mouse models
We then tested the efficacy of CAR+CD45RA– cells in a model mimicking refractory leukemia, in which SEM cells were injected into the mice intravenously several weeks in advance to allow time for the development of intense bioluminescence before effector cell therapy. We found that the signals began to increase starting from day 7 and were quite intense on day 18. Therefore, CAR+CD45RA– cells were injected intravenously on day 18. Thereafter, the signals of bioluminescence rapidly declined and reached an undetectable level in 2 weeks (by day 31; Figure 4f). At the end of the study on day 96, all treated mice remained signal-free. The kinetics of the bioluminescence signals before and after CAR+CD45RA– therapy are depicted in Figure 4g. All mice in the SEM-alone group died before day 40, whereas all in the CAR+CD45RA– group survived (Figure 4h, P<0.001 compared with SEM alone).
We further tested in a mouse model the ability to prevent subsequent relapse, in which the mice that survived prior SEM plus CAR+ cell injections were rechallenged with a second intravenous injection of SEM cells on day 62 after the first inoculum. All five mice survived, with CAR+ cells detectable in the blood but no signs of leukemia or GVHD (Figure 4i, P<0.01 compared with control mice without prior SEM plus CAR+ cell injections).
Memory response of CAR+CD45RA– cells to CMV in vivo
To assess the preservation in CD45RA– cells of the in vivo memory response to common pathogens despite CAR transduction procedures, mice were tested for human CMV recall response using CD45RA+ cells as controls. The two effector cell populations were derived from the same HLA-A*0201+ asymptomatic healthy donors and were injected intraperitoneally. After 1 week, CMV viral lysate-pulsed dendritic cells obtained from the original effector cell donor were injected into the mice intraperitoneally. More cell proliferation was observed in the CD45RA– group than in the CD45RA+ group in the blood 7 days after injection of dendritic cells (Figure 5a). When we analyzed the percentage of proliferating splenocytes that stained positive for CMVpp65-tetramer (defining proliferative cells as BrdU+ in the S phase and BrdUdim7-AADbright in the G2/M phase; Figure 5b), we observed significantly more proliferating CMVpp65-tetramer+ cells in the CD45RA– group after receiving dendritic cells pulsed with CMV (Figure 5c). No such change was observed in the CD45RA+ control group.
The in vivo expansion of CMV-reactive cells in blood and spleen after antigen rechallenge. (a) Fold change in the absolute number of human CD45+ cells in blood after injection of CMV viral lysate-pulsed dendritic cells (DCs). CD45RA– cells or CD45RA+ cells were xenotransplanted to the mice for 1 week. The mice were then given injections with or without viral lysate-pulsed DCs. The absolute human CD45+ cells were measured 1 week later in blood sampling. Data are mean fold change (n=5). (b) Schematic presentation of the gating strategy for the proliferating CMVpp65-tetramer+ cells. The cells were first gated on human CD45 and then analyzed for proliferating cells based on the expression of BrdU (S phase) and 7-aminoactinomycin D (7-AAD; G2/M). The CMVpp65-tetramer+ cells were measured. (c) Percentage of CMVpp65-tetramer+ cells in murine splenocytes from mice of CD45RA-negative and CD45RA-positive cell groups with or without antigen-pulsed DCs. Data are mean±s.e.m. from five mice per group.
CD45RA+ cells were antileukemic but caused GVHD
In our NOG mouse model, CD45RA+ cells with or without CAR could exert strong antileukemia activity (Figure 6a). However, all mice in both groups had severe xenogeneic GVHD with signs of hair loss, diarrhea, dehydration and weight loss. Whereas all mice in the SEM-alone group died of leukemia, all mice in both treatment groups died before day 50 because of GVHD (Figure 6b). Collectively, these data suggested that the antileukemia effect was because of nonspecific alloreactivity (because the effector cells were not human leukocyte antigen (HLA) matched to the SEM cells) and not because of specific anti-CD19 CAR signaling.
CD45RA+ cells with or without CAR were antileukemia but could not prolong survival because of GVHD. (a) Comparison of ventral and dorsal bioluminescence signals among SEM alone (n=10), SEM+CD45RA+ untransduced (n=10) and SEM+CD45RA+ MOI2 groups (n=10). (b) Kaplan–Meier survival curves for SEM alone (n=10), SEM+CD45RA+ untransduced (n=10) and SEM+CD45RA+ MOI2 (n=10). ***P<0.001.
Discussion
Cellular therapy is an attractive modality in cancer treatment, but its many limitations hinder wider application. Currently, cellular therapy is used mostly for hematologic malignancies, especially in patients with multiple relapses, including those for whom prior HCT failed. These diseases are often difficult to cure, and patients are sick with poor organ function or concurrent infections. Here, we described the preclinical use of allogeneic CAR+CD45RA– cells for the treatment of prototypic MLL-rearranged leukemia with three major biological advantages: effective antileukemia and anti-infection activity and no GVHD. The practical advantages include universal availability of a large number of healthy donor cells (because full major histocompatibility complex matching is not required and the cells can be readily obtained by apheresis of original HCT donors or any healthy donors in a non-HCT setting, such as family members), rapid cell processing (requiring only 5 days of cell culture) and efficient transduction (and therefore less demand on vector production requiring an MOI of only 2). Our approach is even possible using off-the-shelf third-party cells, setting a stage for wide clinical application.
Previous CAR-redirected therapy using autologous T cells has shown remarkable clinical efficacy.17, 32, 33, 34, 35, 36, 37 Unfortunately, the preparation of cells typically required 3 weeks or longer, and the T cells from some patients failed to grow, rendering this approach not useful for patients with rapidly progressive leukemia. As an alternative, we and others have pursued allogeneic NK cells as effector cells.38, 39, 40 The cell preparation, however, is similarly difficult, and NK cells are not easy to transduce or cryopreserve. Previously, allogeneic T-cell therapy was limited by the risk of GVHD. Using a simple immunomagnetic cell-separation method, we purified CD45RA– cells and achieved successful separation of GVHD from graft-versus-leukemia and infection immunity both in vitro and in vivo. Based on these preclinical data, CD45RA– cells are being adoptively transferred to our patients after haploidentical HCT to improve immune reconstitution (NCT 01807611). Preliminary clinical data showed that immune reconstitution was rapid, and none of the patients had GVHD despite the infusion of an average of 100 million haplotype-mismatched T cells per kg (W Leung, unpublished data).
In light of the promising data from clinical trials using anti-CD19 CAR-redirected therapy, other investigators have broadened the investigations with various modifications of the CAR constructs and cell engineering processes.8, 12, 15, 41 Although receptor insertion can be mediated using gammaretroviral, lentiviral, mRNA or Sleeping Beauty transposon/transposase systems, it remains to be seen whether nonviral gene delivery systems provide adequate efficacy for CAR-modified T-cell therapy in a clinical setting. Lentiviral vectors provide enhanced transduction efficiency on primary cells and with novel production systems can be produced in a large scale,27, 42 indicating that the method used in this report has potential for wide application. Other than anti-CD19, CAR cells have been generated against GD2 in neuroblastoma,35 CD33/CD123 in myeloid malignancy,11 Her-2 in breast cancer,43 folate receptor-α in ovarian cancer,44 fibroblast activation protein in malignant pleural mesothelioma,45 carboxy-anhydrase-IX in renal cell carcinoma46 and NKG2DL for a broad range of cancers.47, 48 In this study, we generated a lentiviral vector with a MND promoter and the signaling domains of 4-1BB and CD3ζ. Transduction efficiency was high with stable, intense CAR expression during the entire 3-month duration of mouse experiments. Memory response to pathogens common in the cancer population remained robust, suggesting that the transduction procedure did not alter the recall function. The prototypic anti-CD19 backbone can be easily modified to target other tumor-associated molecules as described above.
In addition to using the CAR+CD45RA– cells in the transplant setting (for example, to induce remission before HCT or for the treatment of a relapse after HCT),49, 50 these cells can be used in a non-HCT setting. In this regard, we have previously showed that low-dose cyclophosphamide and fludarabine as a preparative regimen was well tolerated and successfully allowed adoptive transfer of haploidentical NK cells.51 Donor NK cells were detectable for 2–6 weeks after infusion. Thus, the same preparative regimen may be used before the infusion of allogeneic CAR+CD45RA– cells. The CAR+ cells would be expected to last for several weeks after infusion; thereafter, they will be rejected by the recipients when their immunity recovers from the conditioning. If necessary, several cycles of the same treatment may be repeated monthly. Because allogeneic CAR+ cells are not long lasting in the non-HCT setting, long-term toxicity should be limited, and cell-suicide safeguards may not be necessary.
References
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624.
Wayne AS, Giralt S, Kroger N, Bishop MR . Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: introduction. Biol Blood Marrow Transplant 2013; 19: 1534–1536.
Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E . The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 2013; 43: 2797–2809.
Cherel M, Choufi B, Trauet J, Cracco P, Dessaint JP, Yakoub-Agha I et al. Naive subset develops the most important alloreactive response among human CD4 T lymphocytes in HLA-identical related setting. Eur J Haematol 2014; 92: 491–496.
Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood 2007; 109: 3115–3123.
Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood 2008; 111: 2476–2484.
Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest 2003; 112: 101–108.
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013; 121: 1165–1174.
Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010; 16: 1245–1256.
Kochenderfer JN, Rosenberg SA . Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10: 267–276.
Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia 2014; e-pub ahead of print 7 February 2014; doi:10.1038/leu.2014.62.
Dotti G, Gottschalk S, Savoldo B, Brenner MK . Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014; 257: 107–126.
Riddell SR, Jensen MC, June CH . Chimeric antigen receptor—modified T cells: clinical translation in stem cell transplantation and beyond. Biol Blood Marrow Transplant 2013; 19 (1 Suppl): S2–S5.
Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA . The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res 2012; 18: 2780–2790.
Sadelain M, Brentjens R, Riviere I . The basic principles of chimeric antigen receptor design. Cancer Discov 2013; 3: 388–398.
Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra153.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Riet T, Holzinger A, Dorrie J, Schaft N, Schuler G, Abken H . Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol Biol 2013; 969: 187–201.
Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther 2011; 22: 1575–1586.
Singh H, Huls H, Kebriaei P, Cooper LJ . A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev 2014; 257: 181–190.
Barrett DM, Singh N, Porter DL, Grupp SA, June CH . Chimeric antigen receptor therapy for cancer. Annu Rev Med 2014; 65: 333–347.
Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG . Isolation of tumor antigen-specific single-chain variable fragments using a chimeric antigen receptor bicistronic retroviral vector in a Mammalian screening protocol. Hum Gene Ther Methods 2013; 24: 381–391.
Field AC, Vink C, Gabriel R, Al-Subki R, Schmidt M, Goulden N et al. Comparison of lentiviral and sleeping beauty mediated alphabeta T cell receptor gene transfer. PLoS One 2013; 8: e68201.
Tumaini B, Lee DW, Lin T, Castiello L, Stroncek DF, Mackall C et al. Simplified process for the production of anti-CD19-CAR-engineered T cells. Cytotherapy 2013; 15: 1406–1415.
de Boer J, Walf-Vorderwulbecke V, Williams O . In focus: MLL-rearranged leukemia. Leukemia 2013; 27: 1224–1228.
Chan WK, Kung Sutherland M, Li Y, Zalevsky J, Schell S, Leung W . Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands. Clin Cancer Res 2012; 18: 6296–6305.
Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood 2009; 113: 5104–5110.
Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004; 18: 676–684.
Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther 2007; 18: 333–343.
Koldej RM, Carney G, Wielgosz MM, Zhou S, Zhan J, Sorrentino BP et al. Comparison of insulators and promoters for expression of the Wiskott-Aldrich syndrome protein using lentiviral vectors. Hum Gene Ther Clin Dev 2013; 24: 77–85.
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116: 4099–4102.
Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–4828.
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118: 6050–6056.
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra138.
Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P et al. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy 2012; 14: 830–840.
Imai C, Iwamoto S, Campana D . Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005; 106: 376–383.
Cheng M, Chen Y, Xiao W, Sun R, Tian Z . NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10: 230–252.
Jensen MC, Riddell SR . Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 2014; 257: 127–144.
Greene MR, Lockey T, Mehta PK, Kim YS, Eldridge PW, Gray JT et al. Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum Gene Ther Methods 2012; 23: 297–308.
Li S, Yang J, Urban FA, MacGregor JN, Hughes DP, Chang AE et al. Genetically engineered T cells expressing a HER2-specific chimeric receptor mediate antigen-specific tumor regression. Cancer Gene Ther 2008; 15: 382–392.
Kandalaft LE, Powell DJ Jr, Coukos G . A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med 2012; 10: 157.
Schuberth PC, Hagedorn C, Jensen SM, Gulati P, van den Broek M, Mischo A et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med 2013; 11: 187.
Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013; 21: 904–912.
Song DG, Ye Q, Santoro S, Fang C, Best A, Powell DJ Jr . Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther 2013; 24: 295–305.
Zhang T, Sentman CL . Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T cells. J Immunol 2013; 190: 2455–2463.
Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013; 122: 2965–2973.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013; 122: 4129–4139.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010; 28: 955–959.
Acknowledgements
We thank David Galloway for scientific editing the manuscript. This study is supported in part by NCI Cancer Center Core Grant (CA21765) and American Lebanese Syrian Associated Charities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Chan, W., Suwannasaen, D., Throm, R. et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia 29, 387–395 (2015). https://doi.org/10.1038/leu.2014.174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.174
This article is cited by
-
Development of a cGMP-compliant process to manufacture donor-derived, CD45RA-depleted memory CD19-CAR T cells
Gene Therapy (2023)
-
Allogeneic Chimeric Antigen Receptor Therapy in Lymphoma
Current Treatment Options in Oncology (2022)
-
NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma
Blood Cancer Journal (2021)
-
Improved survival rate in T-cell depleted haploidentical hematopoietic cell transplantation over the last 15 years at a single institution
Bone Marrow Transplantation (2020)
-
Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf
BioDrugs (2017)