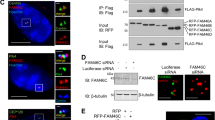
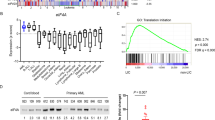
Abstract
Phosphorylation by Akt on Ser 280 was reported to induce cytoplasmic retention and inactivation of CHK1 with consequent genetic instability in PTEN−/− cells. In acute myeloid leukemia cells carrying the FLT3-internal tandem duplication (ITD) mutation, we observed high rates of FLT3-ITD-dependent CHK1 Ser 280 phosphorylation. Pharmacological inhibition and RNA interference identified Pim1/2, not Akt, as effectors of this phosphorylation. Pim1 catalyzed Ser 280 phosphorylation in vitro and ectopic expression of Pim1/2-induced CHK1 phosphorylation. Ser 280 phosphorylation did not modify CHK1 localization, but facilitated its cell cycle and resistance functions in leukemic cells. FLT3, PIM or CHK1 inhibitors synergized with DNA-damaging agents to induce apoptosis, allowing cells to bypass the etoposide-induced G2/M arrest. Consistently, etoposide-induced CHK1-dependent phosphorylations of CDC25C on Ser 216 and histone H3 on Thr11 were decreased upon FLT3 inhibition. Accordingly, ectopic expression of CHK1 improved the resistance of FLT3-ITD cells and maintained histone H3 phosphorylation in response to DNA damage, whereas expression of unphosphorylated Ser 280Ala mutant did not. Finally, FLT3- and Pim-dependent phosphorylation of CHK1 on Ser 280 was confirmed in primary blasts from patients. These results identify a new pathway involved in the resistance of FLT3-ITD leukemic cells to genotoxic agents, and they constitute the first report of CHK1 Ser 280 regulation in myeloid malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dai Y, Grant S . New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res 2010; 16: 376–383.
Petermann E, Maya-Mendoza A, Zachos G, Gillespie DA, Jackson DA, Caldecott KW . Chk1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol 2006; 26: 3319–3326.
Petermann E, Woodcock M, Helleday T . Chk1 promotes replication fork progression by controlling replication initiation. Proc Natl Acad Sci USA 2010; 107: 16090–16095.
Syljuasen RG, Sørensen CS, Hansen LT, Fugger K, Lundin C, Johansson F et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol 2005; 25: 3553–3562.
Krämer A, Mailand N, Lukas C, Syljuåsen RG, Wilkinson CJ, Nigg EA et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol 2004; 6: 884–891.
Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC et al. Chk1 is required for spindle checkpoint function. Dev Cell 2007; 12: 247–260.
Kasahara K, Goto H, Enomoto M, Tomono Y, Kiyono T, Inagaki M . 14-3-3gamma mediates Cdc25A proteolysis to block premature mitotic entry after DNA damage. EMBO J 2010; 29: 2802–2812.
Xu N, Libertini S, Black EJ, Lao Y, Hegarat N, Walker M, Gillespie DA . Cdk-mediated phosphorylation of Chk1 is required for efficient activation and full checkpoint proficiency in response to DNA damage. Oncogene 2012; 31: 1086–1094.
Enomoto M, Goto H, Tomono Y, Kasahara K, Tsujimura K, Kiyono T et al. Novel positive feedback loop between Cdk1 and Chk1 in the nucleus during G2/M transition. J Biol Chem 2009; 284: 34223–34230.
King FW, Skeen J, Hay N, Shtivelman E . Inhibition of Chk1 by activated PKB/Akt. Cell Cycle 2004; 3: 634–637.
Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 2005; 7: 193–204.
Pabla N, Bhatt K, Dong Z . Checkpoint kinase 1 (Chk1)-short is a splice variant and endogenous inhibitor of Chk1 that regulates cell cycle and DNA damage checkpoints. Proc Natl Acad Sci USA 2012; 109: 197–202.
Chung KY, Morrone G, Schuringa JJ, Wong B, Dorn DC, Moore MA . Enforced expression of an Flt3 internal tandem duplication in human CD34+ cells confers properties of self-renewal and enhanced erythropoiesis. Blood 2005; 105: 77–84.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000; 19: 624–631.
Kiyoi H, Naoe T . FLT3 in human hematologic malignancies. Leuk Lymphoma 2002; 43: 1541–1547.
Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood 2003; 101: 3164–3173.
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood 2005; 105: 1759–1767.
Grundler R, Brault L, Gasser C, Bullock AN, Dechow T, Woetzel S et al. Dissection of PIM serine/threonine kinases in FLT3-ITD-induced leukemogenesis reveals PIM1 as regulator of CXCL12-CXCR4-mediated homing and migration. J Exp Med 2009; 206: 1957–1970.
Nawijn MC, Alendar A, Berns A . For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer 2011; 11: 23–34.
Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 2009; 114: 1618–1627.
Hospital MA, Green AS, Lacombe C, Mayeux P, Bouscary D, Tamburini J . The FLT3 and Pim kinases inhibitor SGI-1776 preferentially target FLT3-ITD AML cells. Blood 2012; 119: 1791–1792.
Brault L, Gasser C, Bracher F, Huber K, Knapp S, Schwaller J . PIM serine/threonine kinases in the pathogenesis and therapy of hematologic malignancies and solid cancers. Haematologica 2010; 95: 1004–1015.
Amico D, Barbui AM, Erba E, Rambaldi A, Introna M, Golay J . Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: role of Chk1 and Chk2 phosphorylation and caspase 3. Blood 2003; 101: 4589–4597.
Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M et al. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood 2006; 107: 2517–2524.
Didier C, Cavelier C, Quaranta M, Galcera MO, Demur C, Laurent G et al. G2/M checkpoint stringency is a key parameter in the sensitivity of AML cells to genotoxic stress. Oncogene 2008; 27: 3811–3820.
Cavelier C, Didier C, Prade N, Mansat-De Mas V, Manenti S, Recher C et al. Constitutive activation of the DNA damage signaling pathway in acute myeloid leukemia with complex karyotype: potential importance for checkpoint targeting therapy. Cancer Res 2009; 69: 8652–8661.
Didier C, Demur C, Grimal F, Jullien D, Manenti S, Ducommun B . Evaluation of checkpoint kinase targeting therapy in acute myeloid leukemia with complex karyotype. Cancer Biol Ther 2012; 13: 307–313.
Furet P, Bold G, Meyer T, Roesel J, Guagnano V . Aromatic interactions with phenylalanine 691 and cysteine 828: a concept for FMS-like tyrosine kinase-3 inhibition. Application to the discovery of a new class of potential antileukemia agents. J Med Chem 2006; 49: 4451–4454.
Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, Nakanishi M . Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 2008; 132: 221–232.
Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood 2012; 120: 4205–4214.
Xu N, Hegarat N, Black EJ, Scott MT, Hochegger H, Gillespie DA . Akt/PKB suppresses DNA damage processing and checkpoint activation in late G2. J Cell Biol 2010; 190: 297–305.
Tonic I, Yu WN, Park Y, Chen CC, Hay N . Akt activation emulates Chk1 inhibition and Bcl2 overexpression and abrogates G2 cell cycle checkpoint by inhibiting BRCA1 foci. J Biol Chem 2010; 285: 23790–23798.
Li P, Goto H, Kasahara K, Matsuyama M, Wang Z, Yatabe Y et al. P90 RSK arranges Chk1 in the nucleus for monitoring of genomic integrity during cell proliferation. Mol Biol Cell 2012; 23: 1582–1592.
Acknowledgements
We are very grateful to Dr Cécile Demur and Professor Eric Delabesse for managing and characterization of primary samples. We also thank Dr Shuchi Agrawal-Singh (Copenhagen University) and Dr Ramon Parsons (Columbia University), for kindly providing us with the Pim and CHK1 expression plasmids respectively. We thank Fatima L’Faqihi for technical assistance at the cytometry and cell sorting facility of INSERM 1043, Toulouse. This work was supported by the Association GAEL (Gael Adolescent Espoir Leucémie), by the Institut National de la Santé et de la Recherche Médicale (INSERM), by the Centre National de la Recherche Scientifique (CNRS), by the Association pour la Recherche contre le Cancer (ARC, grant SFI20101201865), by the Institut National du Cancer (INCA, PL2008 INCA-Gov-1345), by the association Inna Biosanté, by the Laboratoire d'Excellence Toulouse Cancer LABEX TOUCAN (Integrative analysis of resistance in hematological cancers) and by the association Laurette Fugain.
Author Contributions
Ling Li Yuan is a recipient of the China Scholarship Council and of the Société Française d’Hématologie (SFH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Yuan, L., Green, A., Bertoli, S. et al. Pim kinases phosphorylate Chk1 and regulate its functions in acute myeloid leukemia. Leukemia 28, 293–301 (2014). https://doi.org/10.1038/leu.2013.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.168
Keywords
This article is cited by
-
Fms-like tyrosine kinase 3-internal tandem duplications epigenetically activates checkpoint kinase 1 in acute myeloid leukemia cells
Scientific Reports (2021)
-
PIM1 accelerates prostate cancer cell motility by phosphorylating actin capping proteins
Cell Communication and Signaling (2020)
-
Targeting the Pim kinases in multiple myeloma
Blood Cancer Journal (2015)
-
DNA damage accumulation and repair defects in acute myeloid leukemia: implications for pathogenesis, disease progression, and chemotherapy resistance
Chromosoma (2014)