Abstract
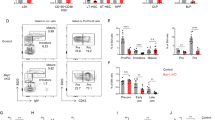
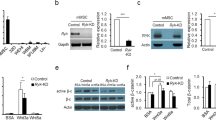
Hematopoiesis is regulated by components of the microenvironment, so-called niche. Here, we show that p190-B GTPase-activating protein (p190-B) deletion in mice causes hematopoietic failure during ontogeny, in p190-B−/− fetal liver and bones, and in p190-B+/− adult bones and spleen. These defects are non-cell autonomous, as we previously showed that transplantation of p190-B−/− hematopoietic cells into wild-type (WT) hosts leads to normal hematopoiesis. Coculture of mesenchymal stem (MSC)/progenitor cells and wild-type bone marrow (BM) cells reveals that p190-B−/− MSCs are dysfunctional in supporting hematopoiesis owing to impaired Wnt signaling. Furthermore, p190-B loss causes alteration in BM niche composition, including abnormal colony-forming unit (CFU)-fibroblast, CFU-adipocyte and CFU-osteoblast numbers. This is due to altered MSC lineage fate specification to osteoblast and adipocyte lineages. Thus, p190-B organizes a functional mesenchymal/microenvironment for normal hematopoiesis during development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shiozawa Y, Havens AM, Pienta KJ, Taichman RS . The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 2008; 22: 941–590.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116: 1195–201.
Kiel MJ, Morrison SJ . Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol 2008; 8: 290–301.
Mikkola HK, Orkin SH . The journey of developing hematopoietic stem cells. Development 2006; 133: 3733–44.
Caplan AI . Mesenchymal stem cells. J Orthop Res 1991; 9: 641–50.
Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV . Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17: 331–40.
Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T . Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med 2002; 195: 1549–63.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007; 131: 324–36.
Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 2009; 457: 490–4.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–34.
Lecourt S, Vanneaux V, Leblanc T, Leroux G, Ternaux B, Benbunan M et al. Bone marrow microenvironment in fanconi anemia: a prospective functional study in a cohort of fanconi anemia patients. Stem Cells Dev 2010; 19: 203–8.
Dror Y, Freedman MH . Shwachman-Diamond syndrome: an inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood 1999; 94: 3048–54.
Mulloy JC, Cancelas JA, Filippi M-D, Kalfa T, Guo F, Zheng Y . Rho GTPases in hematopoiesis and hemopathies. Blood 2010; 115: 936–47.
Hall A . G proteins and small GTPases: distant relatives keep in touch. Science 1998; 280: 2074–2075.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS . Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6: 483–95.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841–6.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ . Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009; 460: 259–63.
Burbelo PD, Miyamoto S, Utani A, Brill S, Yamada KM, Hall A et al. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J Biol Chem 1995; 270: 30919–26.
Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM . p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol 2003; 17: 1054–65.
Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B et al. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell 2002; 2: 553–65.
Sordella R, Jiang W, Chen GC, Curto M, Settleman J . Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 2003; 113: 147–58.
Xu H, Eleswarapu S, Geiger H, Szczur K, Daria D, Zheng Y et al. Loss of the Rho GTPase activating protein p190-B enhances hematopoietic stem cell engraftment potential. Blood 2009; 114: 3557–66.
Geiger H, True JM, Grimes B, Carroll EJ, Fleischman RA, Van Zant G . Analysis of the hematopoietic potential of muscle-derived cells in mice. Blood 2002; 100, p 721–3.
Zhang J, Socolovsky M, Gross AW, Lodish HF . Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 2003; 102: 3938–46.
Wolf NS, Bertoncello I, Jiang D, Priestley G . Developmental hematopoiesis from prenatal to young-adult life in the mouse model. Exp Hematol 1995; 23: 142–6.
Kim JA, Kang YJ, Park G, Kim M, Park YO, Kim H et al. Identification of a stroma-mediated Wnt/beta-catenin signal promoting self-renewal of hematopoietic stem cells in the stem cell niche. Stem Cells 2009; 27: 1318–29.
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J . The pleiotropic effects of the SDF-1–CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006; 20: 1915–1924.
Chasis JA, Mohandas N . Erythroblastic islands: niches for erythropoiesis. Blood 2008; 112: 470–8.
Walkley CR . Erythropoiesis, anemia and the bone marrow microenvironment. Int J Hematol 2011; 93: 10–3.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–41.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL . Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004; 103: 3258–64.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7.
Badillo AT, Flake AW . The regulatory role of stromal microenvironments in fetal hematopoietic ontogeny. Stem Cell Rev 2006; 2: 241–6.
Bianco P . Bone and the hematopoietic niche: a tale of two stem cells. Blood 2011; 117: 5281–8.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–61.
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005; 106: 1232–9.
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 2005; 201: 1781–91.
Ding L, Saunders TL, Enikolopov G, Morrison SJ . Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481: 457–62.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–97.
Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006; 12: 657–64.
Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283: 845–8.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382: 635–8.
Luis TC, Naber BA, Fibbe WE, van Dongen JJ, Staal FJ . Wnt3a nonredundantly controls hematopoietic stem cell function and its deficiency results in complete absence of canonical Wnt signaling. Blood 2010; 116: 496–7.
Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 2009; 113: 546–54.
Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 2011; 9: 345–56.
Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT . Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2008; 2: 274–83.
Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA . Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood 2011; 118: 2420–9.
Kawano Y, Kypta R . Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116 (Pt 13): 2627–34.
Renstrom J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A et al. Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell 2009; 5: 157–67.
Trompouki E, Bowman TV, Lawton LN, Fan ZP, Wu DC, DiBiase A et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 2011; 147: 577–89.
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA . Inhibition of adipogenesis by Wnt signaling. Science 2000; 289: 950–3.
Hill CS, Wynne J, Treisman R . The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 1995; 81: 1159–70.
Beqaj S, Jakkaraju S, Mattingly RR, Pan D, Schuger L . High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J Cell Biol 2002; 156: 893–903.
Kim JM, Choi JS, Kim YH, Jin SH, Lim S, Jang HJ, Kim KT, Ryu SH, Suh PG . An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol 2013; 228: 617–26.
Fonar Y, Gutkovich YE, Root H, Malyarova A, Aamar E, Golubovskaya VM, Elias S, Elkouby YM, Frank D . Focal adhesion kinase protein regulates Wnt3a gene expression to control cell fate specification in the developing neural plate. Mol Biol Cell 2011; 22: 2409–21.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–32.
Claycombe K, King LE, Fraker PJ . A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA 2008; 105: 2017–21.
Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S et al. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood 1997; 90: 3438–43.
Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W . A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 1996; 6: 1170–80.
Axelsson J, Qureshi AR, Heimbürger O, Lindholm B, Stenvinkel P, Bárány P . Body fat mass and serum leptin levels influence epoetin sensitivity in patients with ESRD. Am J Kidney Dis 2005; 46: 628–34.
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000; 100: 197–207.
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005; 434: 514–20.
Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH . Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells 2010; 28: 1071–80.
Matheson SF, Hu KQ, Brouns MR, Sordella R, VanderHeide JD, Settleman J . Distinct but overlapping functions for the closely related p190 RhoGAPs in neural development. Dev Neurosci 2006; 28: 538–50.
Lane SW, De Vita S, Alexander KA, Karaman R, Milsom MD, Dorrance AM et al. Rac signaling in osteoblastic cells is required for normal bone development but is dispensable for hematopoietic development. Blood 2012; 119: 736–44.
Zou W, Greenblatt MB, Shim JH, Kant S, Zhai B, Lotinun S et al. MLK3 regulates bone development downstream of the faciogenital dysplasia protein FGD1 in mice. J Clin Invest 2011; 121: 4383–92.
Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J et al. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J Clin Invest 2010; 120: 1981–93.
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Shao C, Zhao RC . Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009; 23: 925–933.
Ayala F, Dewar R, Kieran M, Kalluri R . Contribution of bone marrow microenvironment to leukemogenesis and leukemia progression. Leukemia 2009; 23: 2233–2241.
Acknowledgements
We thank the mouse core, Jeff Bailey and Victoria Summey, for BM transplantation and the flow cytometry core for assistance with cell sorting at Cincinnati Children’s Hospital Medical Center. The work was supported by NIH (HL090676 and HL104458-MDF).
Author contributions
R Raman designed and performed experiments, analyzed the data and wrote the paper; RS Kumar and A Hinge designed and performed experiments, and analyzed the data; S Kumar, R Nayak, J Xu, K Szczur performed experiments; J A Cancelas provided key advice in research design, data analysis and editing the paper; M-D Filippi designed and directed the program research, analyzed data, and wrote and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Raman, R., Kumar, R., Hinge, A. et al. p190-B RhoGAP regulates the functional composition of the mesenchymal microenvironment. Leukemia 27, 2209–2219 (2013). https://doi.org/10.1038/leu.2013.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.103
Keywords
This article is cited by
-
p190-B RhoGAP and intracellular cytokine signals balance hematopoietic stem and progenitor cell self-renewal and differentiation
Nature Communications (2017)
-
CD147 promotes cell motility via upregulation of p190-B RhoGAP in hepatocellular carcinoma
Cancer Cell International (2016)