Abstract
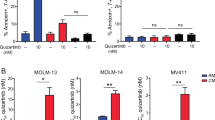
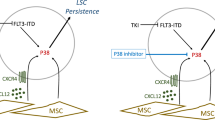
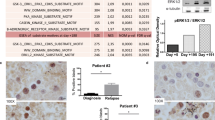
Acute myeloid leukemia (AML) progenitors are frequently characterized by activating mutations in the receptor tyrosine kinase Fms-like tyrosine kinase-3 (FLT3). Protein tyrosine kinases are integral components of signaling cascades that have a role in both FLT3-mediated transformation as well as viability pathways that are advantageous to leukemic cell survival. The bone marrow microenvironment can diminish AML sensitivity to tyrosine kinase inhibitors. We hypothesized that inhibition of protein kinases in addition to FLT3 may be effective in overriding drug resistance in AML. We used a cell-based model mimicking stromal protection as part of an unbiased high-throughput chemical screen to identify kinase inhibitors with the potential to override microenvironment-mediated drug resistance in mutant FLT3-positive AML. Several related multi-targeted kinase inhibitors, including dasatinib, with the capability of reversing microenvironment-induced resistance to FLT3 inhibition were identified and validated. We validated synergy in vitro and demonstrated effective combination potential in vivo. In particular Janus kinase inhibitors were effective in overriding stromal protection and potentiating FLT3 inhibition in primary AML and cell lines. These results hint at a novel concept of using combination therapy to override drug resistance in mutant FLT3-positive AML in the bone marrow niche and suppress or eradicate residual disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitors PKC412. Cancer Cell 2002; 1: 433–443.
Stone RM, DeAngelo DJ, Klimek V, Galinksy I, Estey E, Nimer SD et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 2009; 114: 2984–2992.
Ashley DM, Bol SJ, Kannourakis G . Human bone marrow stromal cell contact and soluble factors have different effects on the survival and proliferation of paediatric B-lineage acute lymphoblastic leukaemic blasts. Leuk Res 1994; 18: 337–346.
Bradstock K, Bianchi A, Makrynikola V, Filshie R, Gottlieb D . Long-term survival and proliferation of precursor B acute lymphoblastic leukemia cells on human bone marrow stroma. Leukemia 1996; 10: 813–820.
Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA . Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma 1997; 27: 375–386.
Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P . Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 1998; 91: 2387–2396.
Lagneaux L, Delforge A, De Bruyn C, Bernier M, Bron D . Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leukemia Lymphoma 1999; 35: 445–453.
Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M . Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia 2002; 16: 1713–1724.
Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009; 113: 4341–4351.
Parmar A, Marz S, Rushton S, Holzwarth C, Lind K, Kayser S et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Res 2011; 71: 4696–4706.
Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther 2008a; 7: 1121–1129.
Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD . FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009; 12: 81–89.
Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood 2006; 108: 2358–2365.
Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM13 and MOLM14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 1997; 11: 1469–1477.
Quentmeier H, Reinhardt J, Zaborski M, Drexler HG . FLT3 mutations in acute myeloid leukemia cell lines. Leukemia 2003; 17: 120–124.
Kimbrel EA, Davis TN, Bradner JE, Kung AL . In vivo pharmacodynamic imaging of proteosome inhibition. Mol Imaging 2009; 8: 140–147.
Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell 2003; 3: 173–183.
Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG . FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 2002; 99: 310–318.
Chou T-C, Talalay P . Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enz. Regul 1984; 22: 27–55.
McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med 2010; 16: 483–489.
Weisberg E, Ray A, Barrett R, Nelson E, Christie AL, Porter D et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia 2010a; 25: 2100–2109.
Weisberg E, Roesel J, Furet P, Bold G, Imbach P, Florsheimer A et al. Antileukemic effects of novel first- and second-generation FLT3 inhibitors: structure-affinity comparison. Genes Cancer 2010b; 1: 1021–1032.
Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG . A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 2005; 23: 329–336.
Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 2011; 29: 1046–1051.
Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A . CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009; 23: 1441–1445.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica 2003; 88: 19–24.
Gu TL, Nardone J, Wang Y, Loriaux M, Villen J, Beausoleil S et al. Survey of activated FLT3 signaling in leukemia. PLoS One 2011; 6: e19169.
Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocyt9ic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 2009; 113: 3050–3058.
Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 2009; 23: 686–697.
Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood 2009; 114: 4441–4450.
Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M et al. Spleen Tyrosine Kinase Is Overexpressed and Represents a Potential Therapeutic Target in Chronic Lymphocytic Leukemia. Cancer Res 2009; 69: 5424–5432.
Steele AJ, Prentice AG, Cwynarski K, Hoffbrand AV, Hart SM, Lowdell MW et al. The JAK3-selective inhibitor PF-956980 reverses the resistance to cytotoxic agents induced by interleukin-4 treatment of chronic lymphocytic leukemia cells: potential for reversal of cytoprotection by the microenvironment. Blood 2010; 116: 4569–4577.
Weisberg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D et al. Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells. Blood 2008b; 111: 3723–3734.
Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther 2007; 6: 1951–1961.
Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia 2011; e-pub ahead of print 20 December 2011.
Summy JM, Gallick GE . Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 2003; 22: 337–358.
Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP . Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 1995; 375: 577–581.
Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA . Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 1999; 4: 915–924.
Kilarski WW, Jura N, Gerwins P . Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp Cell Res 2003; 291: 70–82.
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K et al. Discovery of N-(2-chloro-6-methyl to phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinaes inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004; 47: 6658–6661.
Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL . Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004; 305: 399–401.
Schittenhelm MM, Shiraga S, Schroeder A, Corbin A, Griffith D, Lee FY et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res 2006; 66: 473–481.
Coluccia AM, Cirulli T, Neri P, Mangieri D, Colanardi MC, Gnoni A et al. Validation of PDGFRβ and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood 2008; 112: 1346–1356.
Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S et al. Antitumor activity of targeting Src kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res 2010; 16: 924–935.
Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia 2011; e-pub ahead of print 18 October 2011.
Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood 2003; 101: 3164–3173.
Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W . Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res 2003; 9: 2140–2150.
Robinson LJ, Xue J, Corey SJ . Src family tyrosine kinases are activated by Flt3 and are involved in the proliferative effects of leukemia-associated Flt3 mutations. Exp Hematol 2005; 33: 469–479.
Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood 2006; 108: 1339–1345.
Mony U, Jawad M, Seedhouse C, Russell N, Pallis M . Resistance to FLT3 inhibition in an in vitro model of primary AML cells with a stem cell phenotype in a defined microenvironment. Leukemia 2008; 22: 1395–1401.
Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O'Hare T, Druker BJ, Deininger MW . Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia 2011; e-pub ahead of print 18 November 2011.
Acknowledgements
We wish to thank Feiyang Liu for her technical assistance. We thank DiscoveRx Bioscience for performing KinomeScan profiling and the Treespot view image was generated using the web-based TREEspot software (DiscoveRx Biosciences). JDG is supported by NIH grant CA66996. QL and NSG are supported by NIH LINCS Grant HG006097 and R01 CA130876-02.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
JDG has a financial interest with Novartis Pharma AG. JDG and ALK have a financial interest with Novartis Pharma AG. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Weisberg, E., Liu, Q., Nelson, E. et al. Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors. Leukemia 26, 2233–2244 (2012). https://doi.org/10.1038/leu.2012.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.96
Keywords
This article is cited by
-
Resistance to targeted therapies: delving into FLT3 and IDH
Blood Cancer Journal (2022)
-
Activating JAK-mutations confer resistance to FLT3 kinase inhibitors in FLT3-ITD positive AML in vitro and in vivo
Leukemia (2021)
-
Dasatinib overcomes stroma-based resistance to the FLT3 inhibitor quizartinib using multiple mechanisms
Leukemia (2020)
-
Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells
Nature Communications (2020)
-
Synergistic effect of a novel autophagy inhibitor and Quizartinib enhances cancer cell death
Cell Death & Disease (2018)