Abstract
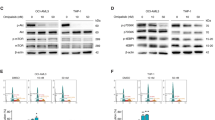
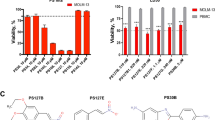
Triptolide, isolated from the herb Tripterygium wilfordii, has been shown to potently induce apoptosis in various malignant cells by inhibiting RNA synthesis and nuclear factor-κB activity. Previously, we showed that triptolide promotes apoptosis in acute myeloid leukemia (AML) cells via the mitochondria-mediated pathway, in part, by decreasing levels of the anti-apoptotic proteins XIAP and Mcl-1. MRx102 is a triptolide derivative, currently in preclinical development. Here we show that MRx102 potently promoted apoptosis in AML cell lines, with EC50 values of 14.5±0.6 nM and 37.0±0.9 nM at 48 h for OCI-AML3 and MV4–11 cells, respectively. MRx102, at low nanomolar concentrations, also induced apoptosis in bulk, CD34+ progenitor, and more importantly, CD34+CD38− stem/progenitor cells from AML patients, even when they were protected by coculture with bone marrow derived mesenchymal stromal cells. MRx102 decreased XIAP and Mcl-1 protein levels and inhibited RNA synthesis in OCI-AML3 cells. In vivo, MRx102 greatly decreased leukemia burden and increased survival time in non-obese diabetic/severe combined immunodeficiency mice harboring Ba/F3-ITD cells. Collectively, we demonstrated that MRx102 has potent antileukemic activity both in vitro and in vivo, has the potential to eliminate AML stem/progenitor cells and overcome microenvironmental protection of leukemic cells, and warrants clinical investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R et al. Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from tripterygium wilfordii. Cancer Lett 1997; 112: 113–117.
Kiviharju TM, Lecane PS, Sellers RG, Peehl DM . Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res 2002; 8: 2666–2674.
Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood 2006; 108: 630–637.
Mak DH, Schober WD, Chen W, Konopleva M, Cortes J, Kantarjian HM et al. Triptolide induces cell death independent of cellular responses to imatinib in blast crisis chronic myelogenous leukemia cells including quiescent CD34+ primitive progenitor cells. Mol Cancer Ther 2009; 8: 2509–2516.
Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S . Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett 1998; 133: 169–175.
Lee KY, Chang W, Qiu D, Kao PN, Rosen GD . PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem 1999; 274: 13451–13455.
Chang WT, Kang JJ, Lee KY, Wei K, Anderson E, Gotmare S et al. Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J Biol Chem 2001; 276: 2221–2227.
Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC et al. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene 2003; 22: 5427–5435.
Carter BZ, Mak DH, Schober WD, Dietrich MF, Pinilla C, Vassilev LT et al. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood 2008; 111: 3742–3750.
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther 2003; 2: 65–72.
Lee KY, Park JS, Jee YK, Rosen GD . Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med 2002; 34: 462–468.
Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem 1999; 274: 13443–13450.
Westerheide SD, Kawahara TL, Orton K, Morimoto RI . Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem 2006; 281: 9616–9622.
Lou YJ, Jin J . Triptolide down-regulates bcr-abl expression and induces apoptosis in chronic myelogenous leukemia cells. Leuk Lymphoma 2004; 45: 373–376.
Zhang C, Cui GH, Liu F, Wu QL, Chen Y . Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol Sin 2006; 27: 1438–1446.
Pan J . RNA polymerase—an important molecular target of triptolide in cancer cells. Cancer Lett 2010; 292: 149–152.
Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ et al. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther 2009; 8: 2780–2790.
Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol 2011; 7: 182–188.
Lu L, Kanwar J, Schmitt S, Cui QC, Zhang C, Zhao C et al. Inhibition of tumor cellular proteasome activity by triptolide extracted from the Chinese medicinal plant ‘thunder god vine’. Anticancer Res 2011; 31: 1–10.
Kupchan SM, Court WA, Dailey Jr RG, Gilmore CJ, Bryan RF . Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc 1972; 94: 7194–7195.
Fidler JM, Li K, Chung C, Wei K, Ross JA, Gao M et al. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther 2003; 2: 855–862.
Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der BD, van DL et al. Phase i dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer 2009; 45: 1764–1772.
Harousseau JLH, Dombret HD, Pigneux AP, Michallet MM, Brandely MB . Phase I study of F60008, a triptolide derivative, in patients with refractory or relapsing acute leukemias. 13th Congress of the European Hematology Association (EHA) 2008, Ref Type: Abstract.
Ling X, Konopleva M, Zeng Z, Ruvolo VR, Stephens LC, Schober W et al. The novel triterpenoid C-28 methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res 2007; 67: 4210–4218.
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M . Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 2002; 62: 3603–3608.
Martin S, Reutelingsperger C, McGahon A, Rader J, van Schie R, LaFace D et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and ABL. J Exp Med 1995; 182: 1545–1556.
Carter BZ, Mak D, Schober WD, Cabreira-Hansen M, Beran M, McQueen T et al. Regulation of survivin expression through bcr-abl/MAPK cascade: targeting survivin overcomes imatinib resistance and increases imatinib sensitivity in imatinib responsive CML cells. Blood 2006; 107: 1555–1563.
Carter BZ, Mak DH, Shi Y, Schober WD, Wang RY, Konopleva M et al. Regulation and targeting of Eg5, a mitotic motor protein in blast crisis CML: overcoming imatinib resistance. Cell Cycle 2006; 5: 2223–2229.
Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001; 98: 2301–2307.
Fidler J, An J, Musser JH, Mak D, Carter BZ, Andreeff M . Preclinical development of triptolide derivative MRx102 as a therapeutic agent for acute myeloid leukemia. Blood 2010; 11: 1340–1341.
Acknowledgements
We thank Kate J Newberry and Deanna A Alexander for helping with the manuscript preparation. This work was supported in part by grants from the National Institutes of Health (P01 CA055164 and MD Anderson's Cancer Center Support Grant CA016672) and by the Paul and Mary Haas Chair in Genetics to MA and NCI SBIR Contract HHSN261200900061C to MyeloRx LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JMF is an employee of MyeloRx and all the other authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Carter, B., Mak, D., Shi, Y. et al. MRx102, a triptolide derivative, has potent antileukemic activity in vitro and in a murine model of AML. Leukemia 26, 443–450 (2012). https://doi.org/10.1038/leu.2011.246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.246
Keywords
This article is cited by
-
The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells
Journal of Neuroinflammation (2017)
-
The triptolide derivative MRx102 inhibits Wnt pathway activation and has potent anti-tumor effects in lung cancer
BMC Cancer (2016)
-
Preclinical antileukemic activity, toxicology, toxicokinetics and formulation development of triptolide derivative MRx102
Cancer Chemotherapy and Pharmacology (2014)
-
Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells
Tumor Biology (2014)
-
Triptolide-Mediated Inhibition of Interferon Signaling Enhances Vesicular Stomatitis Virus-Based Oncolysis
Molecular Therapy (2013)