Abstract
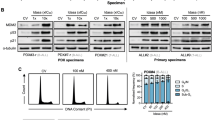
Core-binding factor (CBF) leukemias, characterized by translocations t(8;21) or inv(16)/t(16;16) targeting the CBF, constitute acute myeloid leukemia (AML) subgroups with favorable prognosis. However, about 40% of patients relapse and the current classification system does not fully reflect this clinical heterogeneity. Previously, gene expression profiling (GEP) revealed two distinct CBF leukemia subgroups displaying significant outcome differences and identified apoptotic signaling, MAPKinase signaling and chemotherapy-resistance mechanisms among the most significant differentially regulated pathways. We now tested different inhibitors of the respective pathways in a cell line model (six cell lines reflecting the CBF subgroup-specific gene expression alterations), and found apoptotic signaling to be differentiating between the CBF subgroup models. In accordance, primary samples from newly diagnosed CBF AML patients (n=23) also showed differential sensitivity to in vitro treatment with a Smac mimetic such as BV6, an antagonist of inhibitor of apoptosis (IAP) proteins, and ABT-737, a BCL2 inhibitor. Furthermore, GEP revealed the BV6-resistant cases to resemble the previously identified unfavorable CBF subgroup. Thus, our current findings show deregulated IAP expression and apoptotic signaling to differentiate clinically relevant CBF subgroups, which were independent of known molecular markers, thereby providing a starting point for novel therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dohner K, Dohner H . Molecular characterization of acute myeloid leukemia. Haematologica 2008; 93: 976–982.
Paschka P . Core binding factor acute myeloid leukemia. Semin Oncol 2008; 35: 410–417.
Hart SM, Foroni L . Core binding factor genes and human leukemia. Haematologica 2002; 87: 1307–1323.
Speck NA . Core binding factor and its role in normal hematopoietic development. Curr Opin Hematol 2001; 8: 192–196.
Castilla LH, Perrat P, Martinez NJ, Landrette SF, Keys R, Oikemus S et al. Identification of genes that synergize with Cbfb-MYH11 in the pathogenesis of acute myeloid leukemia. Proc Natl Acad Sci USA 2004; 101: 4924–4929.
Appelbaum FR, Kopecky KJ, Tallman MS, Slovak ML, Gundacker HM, Kim HT et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol 2006; 135: 165–173.
Bullinger L, Rucker FG, Kurz S, Du J, Scholl C, Sander S et al. Gene-expression profiling identifies distinct subclasses of core binding factor acute myeloid leukemia. Blood 2007; 110: 1291–1300.
Marcucci G, Mrozek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol 2005; 23: 5705–5717.
Schlenk RF, Benner A, Krauter J, Buchner T, Sauerland C, Ehninger G et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol 2004; 22: 3741–3750.
Luck SC, Russ AC, Du J, Gaidzik V, Schlenk RF, Pollack JR et al. KIT mutations confer a distinct gene expression signature in core binding factor leukaemia. Br J Haematol 2010; 148: 925–937.
Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol 2006; 24: 3904–3911.
Danial NN, Korsmeyer SJ . Cell death: critical control points. Cell 2004; 116: 205–219.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Gyrd-Hansen M, Meier P . IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 2010; 10: 561–574.
Fulda S . Inhibitor of apoptosis proteins in hematological malignancies. Leukemia 2009; 23: 467–476.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
Vogler M, Dinsdale D, Dyer MJ, Cohen GM . Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ 2009; 16: 360–367.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Pratilas CA, Solit DB . Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res 2010; 16: 3329–3334.
Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R . PAK signaling in oncogenesis. Oncogene 2009; 28: 2545–2555.
Malumbres M, Barbacid M . Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153–166.
Eisen MB, Spellman PT, Brown PO, Botstein D . Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998; 95: 14863–14868.
Alli E, Yang JM, Ford JM, Hait WN . Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol 2007; 71: 1233–1240.
Viaud J, Peterson JR . An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther 2009; 8: 2559–2565.
Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest 2001; 108: 851–859.
Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004; 428: 198–202.
Risinger AL, Giles FJ, Mooberry SL . Microtubule dynamics as a target in oncology. Cancer Treat Rev 2009; 35: 255–261.
Bliss C . The toxicity of poisons applied jointly. Ann Appl Biol 1939; 26: 585–615.
Greco WR, Bravo G, Parsons JC . The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995; 47: 331–385.
Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 2007; 12: 445–456.
High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O’Brien R, Khaw SL et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol 2010; 77: 483–494.
Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006; 10: 375–388.
Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res 2008; 68: 2321–2328.
Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res 2007; 67: 1176–1183.
Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA et al. Seed analysis of off-target siRNAs reveals an essential role of Mcl-1in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene 2007; 26: 3972–3979.
Yecies D, Carlson NE, Deng J, Letai A . Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010; 115: 3304–3313.
Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther 2010; 9: 545–557.
Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B . An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res 2009; 15: 3172–3176.
O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009; 113: 299–305.
Wilson WH, O’Connor OA, Czuczman MS, Lacasce AS, Gerecitano JF, Leonard JP et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010; 11: 1149–1159.
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 1993; 81: 3091–3096.
Tothova E, Fricova M, Stecova N, Kafkova A, Elbertova A . High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Neoplasma 2002; 49: 141–144.
Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH . cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res 2009; 69: 1782–1791.
Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, Xue W et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol 2008; 10: 1309–1317.
Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J . RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ 2010; 17: 482–487.
Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 2008; 30: 689–700.
Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ 2010; 18: 656–665.
LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG . IAP-targeted therapies for cancer. Oncogene 2008; 27: 6252–6275.
Acknowledgements
We thank Sabine Renz and Anna Siegmund for excellent technical assistance with Ficoll gradient separation of primary CBF AML samples. We are grateful for Juan Du's help in establishing mutational screening assays. Furthermore, BV6 was kindly supplied by Genentech. This study was supported in part by the Deutsche José Carreras Stiftung e.V. (DJCLS R 08/32f) and the German Research Foundation (DFG DO 704/3-1); LB was supported in part by the German Research Foundation (Heisenberg-Stipendium BU 1339/3-1) and SF was supported in part by the German Research Foundation, Deutsche José Carreras Stiftung e.V., the EU (ApopTrain, APO-SYS) and IAP16/8.
Author contributions
SCL designed the research, performed research, analyzed and interpreted data, and wrote the manuscript. ACR designed the research, collected, analyzed and interpreted data, and reviewed the manuscript. UB, PP and RFS collected data and reviewed the manuscript. HD and KD designed research and reviewed the manuscript. SF designed the research, contributed vital reagents, analyzed data and reviewed the manuscript. LB designed the research, interpreted data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Lück, S., Russ, A., Botzenhardt, U. et al. Deregulated apoptosis signaling in core-binding factor leukemia differentiates clinically relevant, molecular marker-independent subgroups. Leukemia 25, 1728–1738 (2011). https://doi.org/10.1038/leu.2011.154
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.154
Keywords
This article is cited by
-
Multi-study reanalysis of 2,213 acute myeloid leukemia patients reveals age- and sex-dependent gene expression signatures
Scientific Reports (2019)
-
Bypassing drug resistance by triggering necroptosis: recent advances in mechanisms and its therapeutic exploitation in leukemia
Journal of Experimental & Clinical Cancer Research (2018)
-
The cell fate determinant Scribble is required for maintenance of hematopoietic stem cell function
Leukemia (2018)
-
Epo-induced erythroid maturation is dependent on Plcγ1 signaling
Cell Death & Differentiation (2015)
-
Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities
Leukemia (2014)