Abstract
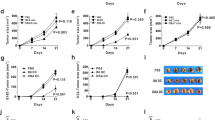
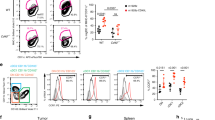
Tumor escape mechanisms in leukemia are not well defined. To dissect immunological mechanisms responsible for immune tolerance toward leukemia, we established a murine model system allowing clonotypic analysis of leukemia-specific CD4 T cells recognizing ovalbumin (OVA). Upon i.v. injection of genetically engineered leukemia cells, dendritic cells (DCs) engulfed, processed and presented OVA to OVA-specific CD4 T cells. Consequently, leukemia-specific T cells were primed in vivo as shown by expression of activation markers and proliferative responses. However, in spite of detectable CD4 T cell responses in vitro and in vivo, no effective anti-leukemia immunity was established. In contrast, adoptively transferred DO11.10 T cells that were primed ex vivo mediated effective antitumor immunity. Furthermore, ex vivo primed DO11.10 T cells showed high expression of Th1 cytokines (interferon-γ, tumor necrosis factor-α and interleukin-2) whereas in vivo primed OVA-specific CD4 T cells showed incomplete differentiation (proliferation without cytokine production). We conclude that activated T cells lacking effector function develop through incomplete differentiation in leukemia-bearing mice. Thus, priming conditions of leukemia-specific CD4 T cells critically determines the balance between immunity or tolerance toward leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Street SE, Cretney E, Smyth MJ . Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001; 97: 192–197.
Street SE, Trapani JA, MacGregor D, Smyth MJ . Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med 2002; 196: 129–134.
Mitra-Kaushik S, Harding J, Hess J, Schreiber R, Ratner L . Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood 2004; 104: 3305–3311.
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 1998; 95: 7556–7561.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410: 1107–1111.
van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med 1996; 184: 1781–1790.
Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA . Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 2000; 192: 755–760.
Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 2000; 191: 661–668.
Smyth MJ, Crowe NY, Godfrey DI . NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 2001; 13: 459–463.
Girardi M, Glusac E, Filler RB, Roberts SJ, Propperova I, Lewis J et al. The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. J Exp Med 2003; 198: 747–755.
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294: 605–609.
Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med 2003; 198: 433–442.
Zheng P, Sarma S, Guo Y, Liu Y . Two mechanisms for tumor evasion of preexisting cytotoxic T-cell responses: lessons from recurrent tumors. Cancer Res 1999; 59: 3461–3467.
Uyttenhove C, Maryanski J, Boon T . Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med 1983; 157: 1040–1052.
Singh S, Ross SR, Acena M, Rowley DA, Schreiber H . Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med 1992; 175: 139–146.
Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA 1999; 96: 2233–2238.
Shimizu J, Yamazaki S, Sakaguchi S . Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999; 163: 5211–5218.
Gabrilovich D . Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 2004; 4: 941–952.
Greenberg PD, Cheever MA, Fefer A . Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2- lymphocytes. J Exp Med 1981; 154: 952–963.
Romerdahl CA, Kripke ML . Role of helper T-lymphocytes in rejection of UV-induced murine skin cancers. Cancer Res 1988; 48: 2325–2328.
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H . The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188: 2357–2368.
Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD et al. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci USA 1999; 96: 8633–8638.
Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005; 22: 371–383.
Murphy KM, Heimberger AB, Loh DY . Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 1990; 250: 1720–1723.
Stripecke R, Skelton DC, Gruber T, Afar D, Pattengale PK, Witte ON et al. Immune response to Philadelphia chromosome-positive acute lymphoblastic leukemia induced by expression of CD80, interleukin 2, and granulocyte-macrophage colony-stimulating factor. Hum Gene Ther 1998; 9: 2049–2062.
Klein C, Bueler H, Mulligan RC . Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med 2000; 191: 1699–1708.
Hawley TS, Fong AZ, Griesser H, Lyman SD, Hawley RG . Leukemic predisposition of mice transplanted with gene-modified hematopoietic precursors expressing flt3 ligand. Blood 1998; 92: 2003–2011.
Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P . The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med 1983; 157: 1149–1169.
Lyons AB, Parish CR . Determination of lymphocyte division by flow cytometry. J Immunol Methods 1994; 171: 131–137.
Stripecke R, Skelton DC, Pattengale PK, Shimada H, Kohn DB . Combination of CD80 and granulocyte-macrophage colony-stimulating factor coexpression by a leukemia cell vaccine: preclinical studies in a murine model recapitulating Philadelphia chromosome-positive acute lymphoblastic leukemia. Hum Gene Ther 1999; 10: 2109–2122.
Gruber TA, Skelton DC, Kohn DB . Requirement for NK cells in CD40 ligand-mediated rejection of Philadelphia chromosome-positive acute lymphoblastic leukemia cells. J Immunol 2002; 168: 73–80.
Toes RE, Ossendorp F, Offringa R, Melief CJ . CD4 T cells and their role in antitumor immune responses. J Exp Med 1999; 189: 753–756.
Greenberg PD, Kern DE, Cheever MA . Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med 1985; 161: 1122–1134.
Frey AB . Rat mammary adenocarcinoma 13762 expressing IFN-gamma elicits antitumor CD4+ MHC class II-restricted T cells that are cytolytic in vitro and tumoricidal in vivo. J Immunol 1995; 154: 4613–4622.
Wang LX, Shu S, Disis ML, Plautz GE . Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (TEs) combined with CD8+ TEs provides intratumoral TE proliferation and synergistic antitumor response. Blood 2007; 109: 4865–4876.
Schild HJ, Kyewski B, Von Hoegen P, Schirrmacher V . CD4+ helper T cells are required for resistance to a highly metastatic murine tumor. Eur J Immunol 1987; 17: 1863–1866.
Hock H, Dorsch M, Diamantstein T, Blankenstein T . Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med 1991; 174: 1291–1298.
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993; 90: 3539–3543.
Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ . Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998; 187: 693–702.
Marzo AL, Lake RA, Robinson BW, Scott B . T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res 1999; 59: 1071–1079.
Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW et al. Tumor-specific CD4+ T cells have a major ‘post-licensing’ role in CTL mediated anti-tumor immunity. J Immunol 2000; 165: 6047–6055.
Levitsky HI, Lazenby A, Hayashi RJ, Pardoll DM . In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med 1994; 179: 1215–1224.
Tanaka Y, Koido S, Xia J, Ohana M, Liu C, Cote GM et al. Development of antigen-specific CD8+ CTL in MHC class I-deficient mice through CD4 to CD8 conversion. J Immunol 2004; 172: 7848–7858.
Xiang J, Huang H, Liu Y . A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol 2005; 174: 7497–7505.
Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D . Immune response to green fluorescent protein: implications for gene therapy. Gene Therapy 1999; 6: 1305–1312.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD . Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–998.
Qin Z, Blankenstein T . CD4+ T cell—mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity 2000; 12: 677–686.
Cloppenborg T, Stanulla M, Zimmermann M, Schrappe M, Welte K, Klein C . Immunosurveillance of childhood ALL: polymorphic interferon-gamma alleles are associated with age at diagnosis and clinical risk groups. Leukemia 2005; 19: 44–48.
Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149.
Okano F, Merad M, Furumoto K, Engleman EG . In vivo manipulation of dendritic cells overcomes tolerance to unmodified tumor-associated self antigens and induces potent antitumor immunity. J Immunol 2005; 174: 2645–2652.
Acknowledgements
We thank Renata Stripecke for BM185 cells, Thomas Kamradt for DO11.10 mice, Matthias Ballmaier for cell sorting, Inga Sandrock for excellent technical support, Ricardo Dewey and Chozhavendan Rathinam for helpful discussions and Karl Welte for continuous support. This study was supported by the Wilhelm-Sander-Foundation 0172 5431807 and DFG (SFB738).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Hegazy, A., Klein, C. Ex vivo priming of CD4 T cells converts immunological tolerance into effective antitumor immunity in a murine model of acute lymphoblastic leukemia. Leukemia 22, 2070–2079 (2008). https://doi.org/10.1038/leu.2008.193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.193
Keywords
This article is cited by
-
Induction of antigen-specific effector-phase tolerance following vaccination against a previously ignored B-cell lymphoma
Immunology and Cell Biology (2011)