Abstract
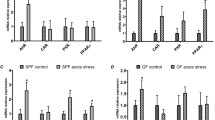
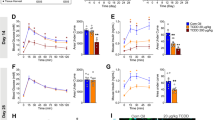
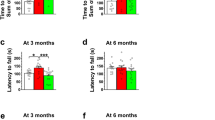
Oral gavage is a widely used method for administering substances to animals in pharmacological and toxicological studies. The authors evaluated whether oral gavage causes behavioral indicators of stress, increased mortality rate, alterations in food and water consumption and body weight or histological lesions in CD-1 mice. Gavage was carried out once per d for 5 d per week over 6 consecutive weeks. The mortality rate of mice in this study was 15%. Mice subjected to gavage did not undergo changes in food or water consumption during the study, and their mean body weights and relative organ weights were similar to those of mice in the control group. Serum cortisol levels at the time of euthanasia in mice in both groups were within the normal range. Histopathology showed acute esophagitis and pleurisy, indicative of perforation of the esophagus, in the two mice that died but no abnormalities in the other mice. The results suggest that animal stress and mortality related to oral gavage can be minimized when the procedure is carried out by an experienced technician.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vasconcelos-Nóbrega, C. et al. In vivo and in vitro effects of RAD001 on bladder cancer. Urol. Oncol. doi 10.1016/j.urolonc.2011.11.002 (published online 9 December 2011).
Arantes-Rodrigues, R. et al. High doses of olive leaf extract induce liver changes in mice. Food. Chem. Toxicol. 49, 1989–1997 (2011).
Morton, D.B. et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW joint working group on refinement. Lab. Anim. 35, 1–41 (2001).
Ferguson, S.A. & Boctor, S.Y. Use of food wafers for multiple daily oral treatments in young rats. J. Am. Assoc. Lab. Anim. Sci. 96, 292–295 (2009).
Suckow, M.A., Danneman, P. & Brayton, C. The Laboratory Mouse (CRC, Boca Raton, FL, 2001).
Murphy, S.J., Smith, P., Shaivitz, A.B., Rossberg, M.I. & Hurn, P.D. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. Contemp. Top. Lab. Anim. Sci. 40, 9–12 (2001).
Fortman, J.D., Hewett, T.A. & Bennet, B.T. The Laboratory Nonhuman Primate (CRC, Boca Raton, FL, 2002).
Atcha, Z. et al. Alternative method of oral dosing for rats. J. Am. Assoc. Lab. Anim. Sci. 49, 335–343 (2010).
Hoggatt, A.F., Hoggatt, J., Honerlaw, M. & Pelus, L.M. A spoonful of sugar helps the medicine go down: a novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 49, 329–334 (2010).
Bennink, R.J. et al. Validation of gastric-emptying scintigraphy of solids and liquids in mice using dedicated animal pinhole scintigraphy. J. Nucl. Med. 44, 1099–1104 (2003).
Dobrakovová, M. & Jurcovicová, J. Corticosterone and prolactin responses to repeated handling and transfer of male rats. Exp. Clin. Endocrinol. 83, 21–27 (1984).
Kramer, K. et al. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab. Anim. 34, 272–280 (2000).
Brown, A.P., Dinger, N. & Levine, B.S. Stress produced by gavage administration in the rat. Contemp. Top. Lab. Anim. Sci. 39, 17–21 (2000).
Balcombe, J.P., Barnard, N.D. & Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 43, 42–51 (2004).
Olfe, J., Domanska, G., Schuett, C. & Kiank, C. Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol. 10, 1–11 (2010).
Loveless, S.E. et al. Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 220, 203–217 (2006).
Loveless, S.E., Hoban, D., Sykes, G., Frame, S.R. & Everds, N.E. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 105, 86–96 (2008).
Germann, P.G., Ockert, D. & Tuch, K. Oropharyngeal granulomas and tracheal cartilage degeneration in Fischer-344 rats. Toxicol. Pathol. 23, 349–355 (1995).
Õkva, K. et al. Refinements for intragastric gavage in rats. Scand. J. Lab. Anim. Sci. 33, 243–252 (2006).
Eichenbaum, G. et al. Impact of gavage dosing procedure and gastric content on adverse respiratory effects and mortality in rat toxicity studies. J. Appl. Toxicol. 31, 342–354 (2010).
Damsch, S. et al. Gavage-related reflux in rats: identification, pathogenesis, and toxicological implications (review). Toxicol. Pathol. 39, 348–360 (2011).
Qu, W.M., Huang, Z.L., Matsumoto, N., Xu, X.H. & Urade, Y. Drug delivery through a chronically implanted stomach catheter improves efficiency of evaluating wake-promoting components. J. Neurosci. Meth. 175, 58–63 (2008).
European Commission. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 and of the council of 22 September 2010 on the protection of animals used for scientific purposes (Brussels, Belgium, 2010).
Wheeler, T.L., Eppolito, A.K., Smith, L.N., Huff, T.B. & Smith, R.F. A novel method for oral stimulant administration in the neonate rat and similar species. J. Neurosci. Meth. 159, 282–285 (2007).
Kaliste, E. The Welfare of Laboratory Animals vol. 2 (ed. Clive Phillips) (Springer, Dordrecht, The Netherlands, 2007).
Moore, M.C. & Palmer, N.G. Calculations for Veterinary Nurses (Blackwell Science, London, UK, 2001).
Foltz, C.J. & Ullman-Cullere, M. Guidelines for assessing the health and condition of mice. Lab Anim. (NY) 28, 28–32 (1999).
Germann, P.G. & Ockert, D. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for the unexpected high mortality in a Fischer 344 rat carcinogenicity study. Lab. Anim. Sci. 44, 338–343 (1994).
Wheatley, J.L. A gavage dosing apparatus with flexible catheter provides a less stressful gavage technique in the rat. Lab Anim. (NY) 31, 53–56 (2002).
Hau, J. & Hoosier, G.V. Handbook of Laboratory Animal Science vol. 1 (CRC, Boca Raton, FL, 2003).
Ullman-Culleré, M.H. & Foltz, C.J. Body condition scoring: a rapid and accurate method for assessing health status in mice. J. Am. Assoc. Lab. Anim. Sci. 49, 319–323 (1999).
Acknowledgements
We thank Mrs. Lígia Lourenço for her technical assistance. We thank Professor Jorge Colaço for his contribution to the statistical analyses. This study was supported by an aid grant from the Fundação para a Ciência e Tecnologia, Ministério da Ciência e Ensino Superior, Portugal (grant number SFRH/BD/47612/2008) and was financed by FCT Pest-OE/AGR/UI0772/2011 unity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Arantes-Rodrigues, R., Henriques, A., Pinto-Leite, R. et al. The effects of repeated oral gavage on the health of male CD-1 mice. Lab Anim 41, 129–134 (2012). https://doi.org/10.1038/laban0512-129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/laban0512-129
This article is cited by
-
Towards more translatable research: Exploring alternatives to gavage as the oral administration route of vaccines in rodents for improved animal welfare and human relevance
Lab Animal (2023)
-
Silk protein sericin: a promising therapy for Achilles tendinopathy—evidence from an experimental rat model
Clinical Rheumatology (2023)
-
Disturbance of Mitochondrial Dynamics, Endoplasmic Reticulum–Mitochondria Crosstalk, Redox Homeostasis, and Inflammatory Response in the Brain of Glutaryl-CoA Dehydrogenase-Deficient Mice: Neuroprotective Effects of Bezafibrate
Molecular Neurobiology (2022)
-
Thymine DNA glycosylase as a novel target for melanoma
Oncogene (2019)