Abstract
Objective:
The excipients benzyl alcohol, propylene glycol and ethanol are present in medications used in the neonatal intensive care unit. Exposure to high levels can have adverse effects in a neonatal population. The objective was to quantify excipient exposure in very low birth weight (VLBW) neonates and identify risk factors associated with greater exposure.
Study design:
A retrospective record review of VLBW infants admitted over 1 year. Excipient exposures were calculated and multivariable regression analyses identified risk factors for increasing exposure.
Results:
In total, 98% of subjects were exposed to at least one excipient. A total of 5 to 9% received doses higher than recommended for adults. Necrotizing enterocolitis, seizure, bronchopulmonary dysplasia and longer stay predicted higher excipient exposure.
Conclusion:
The excipients examined are in medications commonly prescribed for VLBW neonates, and cumulative doses may exceed recommended exposures for adults. Although safety profiles have not been established, judicious use of medication containing these excipients is warranted for this population.
Similar content being viewed by others
Introduction
Excipients are chemicals added to pharmaceutical products to extend shelf life, enhance solubility or absorption, control release of active ingredients and/or increase palatability. They are not intended to exert therapeutic effects. Recent studies show that neonates in hospitals may be exposed to high levels of excipients, often at doses higher than recommended for adults.1, 2, 3, 4 High levels of excipient exposure in neonates have led to adverse effects. In healthy children and adults, both the preservative benzyl alcohol (BA) and the solubilizer propylene glycol (PG) are metabolized rapidly into harmless chemicals: BA is oxidized to benzoic acid, which is conjugated with glycine in the liver, and excreted as hippuric acid, whereas PG is metabolized into pyruvic acid, acetic acid and lactic acid, and excreted in the urine. However, preterm babies are unable to use these metabolic pathways effectively.5 Inefficient metabolism of BA causes accumulation which can result in dyspnea, sedation and loss of motor function. Animal studies have shown that these effects are due to BA itself and not a metabolite.6 Exposure to high levels of BA is associated with developmental delay, cerebral palsy and death in neonates.7, 8, 9, 10 The half-life of PG is three times longer in neonates than in adults,11, 12 and exposure to high levels of PG has been associated with lactic acidosis, central nervous system depression and seizures (for example,13, 14, 15). Another common excipient is ethanol (ET), which is used as a solvent and a preservative. Metabolism of ET may also be immature in neonates16 and at this time there is no known safe level of ET exposure.
Safety data for some excipients are available for adults but not for the preterm population.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA; administered by the Food and Agriculture Organization of the United Nations (FAO) and the WHO) has established the acceptable daily oral intake (ADI) for BA and PG for adults at 0 to 5 mg kg−1 per day and 0 to 25 mg kg−1 per day, respectively.17, 18, 19 No ADI has been established for BA or PG for children. And no amount of ET intake should be considered safe, as any exposure is potentially harmful to the developing brain.20 The AAP (American Academy of Pediatrics) Committee on Drugs recommends that medications intended for children should contain no ET, however, if ET must be utilized it should be limited to a maximum of 5% (v/v) and should not be capable of producing a blood alcohol level >25 mg/dl after a single dose.21
Prior studies report that pediatric patients admitted to intensive care units may be exposed to more than the established ADIs for BA and PG.1 To our knowledge, there is no prior study quantifying exposure to BA, PG and ET in the preterm very low birth weight (VLBW, ⩽1500 g) population. In 2014 ~1.4% of live births, or 55 833 babies, were VLBW.22 These children are at risk for numerous comorbidities related to prematurity and are frequently in need of multiple medications. Thus, there is the potential for exposure to multiple excipients. The aim of this study was to quantify exposure to three excipients: BA, PG and ET, and to identify risk factors associated with highest exposure.
Methods
A retrospective medical record review was performed on charts of VLBW neonates from the University of Maryland Medical Center Level IV neonatal intensive care unit (NICU) born between 1 July 2012 and 30 June 30 2013. The study had prior approval from the Institutional Review Board at the University of Maryland, Baltimore. Inclusion criteria were: birth gestational age (GA) ⩽30 weeks, birth weight (BW) ⩽1500 g and admission to the NICU within 24 h of birth. Infants transferred to our NICU at >24 h were excluded as this could limit access to accurate medication records.
Demographic and clinical data collected included: sex, BW, GA, small for gestational age status, maternal antenatal steroids, resuscitation requirements (including surfactant administration), respiratory support requirements (including increasing severity from room air to nasal cannula to continuous positive airway pressure to conventional mechanical ventilation to high frequency oscillatory ventilation), diagnoses such as: patent ductus arteriosus, necrotizing enterocolitis (NEC) Bell’s stage II or III,23 intraventricular hemorrhage, bronchopulmonary dysplasia (BPD) defined as oxygen requirement +/− positive pressure ventilation at 28 days, and at 36 weeks post menstrual age, Score of Neonatal Acute Physiology Perinatal Extension II (SNAPPE II24, 25), retinopathy of prematurity, length of stay and death prior to discharge.
Data on medications received included route of administration, dose and duration. Data on excipient content of each medication was calculated from information in the packaging inserts or as reported by pharmaceutical companies. Parenteral nutrition, surfactant and inhalant medications did not contain any of the three excipients, and excipient exposure from topical or ophthalmic medications was not calculated owing to variability in the amount applied by different providers. Total excipient exposure was defined as the total amount of excipient received during the period of hospitalization (mg for BA and PG, ml for absolute ET). Weight-based exposures (mg kg−1 per day and ml kg−1 per day) were performed by calculating the cumulative exposure per day and dividing by BW until the subject’s weight surpassed BW, at which time the cumulative exposure was divided by the weight recorded on the Monday of that week.
Demographic and clinical risk factors were analyzed using t-test and χ2 as appropriate for continuous, binary and categorical variables. Multivariable linear regression models were used to determine significant predictors of increasing excipient exposure for BA, PG and ET. Multivariable logistic regression was used to determine significant predictors of any exposure to BA; this was not done for PG or ET as a majority of subjects was exposed to these excipients. Analyses were performed using Microsoft Excel 2010 and SAS 9.3 (Carey, NC, USA).
Results
There were 106 eligible subjects. Mean (s.d.) BW of the overall study cohort was 961 g (245 g), mean GA was 27.4 weeks (2.1 weeks), and 60% of subjects were female. Thirteen subjects (12%) died prior to discharge. Five of these died within the first 48 h of life. Mean SNAPPE II score was significantly (P⩽0.00001) higher in subjects who died compared with those who survived till discharge (73±29 vs 27±20). Demographic and clinical characteristics are shown in Table 1.
Excipient exposure
Nineteen of the 110 per os (PO), intrvenous (IV) or intramuscular preparations contained one or more of the excipients (Table 2). In this cohort, 98% of subjects (n=104) were exposed to at least one of the excipients and 85% received two or more excipients (Tables 1 and 3). Around 11% of subjects were exposed to an excipient at a dose greater than the WHO or FDA recommendations for adults, and ~4% were exposed to more than the adult ADI for both BA and PG for at least one day during their stay. Frequency of exposures to BA, PG and ET were 34% (n=36), 88% (n=93) and 86% (n=91), respectively. Two subjects (1.9%) had no excipient exposure; one died within 48 h, therefore had minimal medication exposure and the other had a birth weight of 1490 g, thus, was at the upper limit of VLBW definition. Approximately 25% of the population (n=26) was exposed to all three excipients, 54% was exposed to both PG and ET, whereas co-exposures of PG or ET with BA were low (~3%; Table 3).
Benzyl alcohol
Eight medications contained BA. IV forms of midazolam (56%), dexamethasone (20%), phenobarbital (14%) and hydrocortisone (8%) were responsible for 98% of BA exposure. Among the 36 subjects exposed to BA, the median exposure was 1.15 mg kg−1 per day (interquartile range 1.8; Table 4).
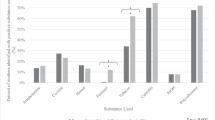
Four subjects (11%) in the BA exposed group received a median daily exposure greater than the WHO ADI for adults. The subject with highest total BA exposure received 922.5 mg over a period of 16 days (7.9 mg kg−1 per day; Figure 1). This subject was male with BW of 610 g and GA 24-6/7 weeks, received 72 different medications and died prior to discharge. Three of the 5 (67%) patients with the highest daily median BA exposure died prior to discharge.
Median daily excipient exposure. Daily median exposure to each excipient for all subjects in the cohort shown in order of increasing exposure. Triangles denote females and squares show males. Dashed lines show acceptable daily intake levels for adults for BA and PG determined by the Joint Food and Agriculture Organization of the United Nations and the World Health Organization Expert Committee on Food Additives.
The multivariable logistic regression model demonstrated that increasing respiratory support requirement, any intraventricular hemorrhage diagnosis, NEC Stage II or III diagnosis, and patent ductus arteriosus diagnosis were associated with increased odds of BA exposure (Table 5). The multivariable linear regression showed that significant predictors of higher BA exposure as a continuous variable were NEC, presence of seizures, BPD at 36 weeks and death prior to discharge (Table 6).
All subjects who received BA required mechanical ventilation at some point during hospitalization; 69% (n=25) were on high frequency oscillatory ventilation and 83% (n=30) required supplemental oxygen at 28 days postnatal life. The mean SNAPPE II score was significantly (P<0.001) higher in subjects exposed to BA compared with the no BA exposure cohort; 49 (±29) vs 23 (±20).
Propylene glycol
PG was present in seven medications used in the NICU. Phenobarbital IV (66%), nystatin PO (25%) and lorazepam IV (6%) accounted for the majority of PG exposure.
Among the 93 babies who were exposed to PG, the median exposure was 6.3 mg kg−1 (interquartile range 3.1; Table 4). Ten subjects (11%) in the PG exposed group received more PG than the WHO ADI for adults. The subject with the longest duration of exposure received 3081 mg over a period of 120 days (25.6 mg kg−1 per day; Figure 1), was a male with BW of 850 g, GA of 28-6/7 weeks and received 53 different medications. This subject died prior to discharge. The subject with the highest overall exposure received 117 mg kg−1 per day over 2 days, was male with BW of 1220 g, GA of 29-6/7 weeks, and received 26 different medications, and survived to discharge.
Multivariable linear regression models demonstrated that significant predictors of being exposed to increasing doses of PG included NEC and seizure diagnoses (Table 6). In this group, 93% (n=87) of subjects required mechanical ventilation, with 29% requiring high frequency oscillatory ventilation.
Ethanol
Ten medications utilized by the cohort contained ET. Oral preparations of potassium chloride (63%), ranitidine (26%) and chlorothiazide (3%) accounted for the majority of the ET exposure. Mean SNAPPE II scores were higher (P=0.003) in those subjects not exposed to ET (60±30) than in those who were exposed (28±22). Twenty-three subjects (26.1%) required high frequency oscillatory ventilation.
For the 91 subjects exposed to ET, the median daily exposure was 0.01 ml kg−1 per day (interquartile range 0.03; Table 4). The subject with the highest ET exposure received 74 ml over a period of 204 days (0.11 ml kg−1 per day; Figure 1). This subject was female, BW 1125 g, GA of 27-6/7 weeks, and received 49 different medications. Multivariable linear regression models demonstrated that significant predictors of exposure to higher doses of ET included longer length of stay and BPD at 36 weeks corrected GA (Table 6).
Discussion
Our cohort was exposed to 110 different medications via IV, intramuscular or PO routes. Nineteen of the medications routinely used in the University of Maryland Medical Center NICU during the time of the study contained one or more of the three excipients of interest. Our data underestimates overall excipient exposure as we did not quantify every excipient contained in these medications, nor did we include medications given by other routes.
Overall, 98% of patients were exposed to at least one of the three excipients. Exposure to PG or ET was >80%, which is in contrast to recent reports from Europe in which risk of exposure to a particular excipient is low; ~10% of extremely or very preterm infants were exposed to either excipient.4, 26 In contrast, rates of excipient exposure in Brazil were similar to that seen in the current study,3 although doses were not reported.
The median exposure of the study cohort to BA was less than the WHO ADI for adults, however, 11% of infants exposed to BA received more than the WHO ADI for adults on at least 1 day. We found that higher BA exposure was related to NEC, seizure diagnosis and BPD. Thus, patients exposed to BA were typically critically ill (as evidenced by the significantly higher SNAPPE II scores) and were more likely to be prescribed medications containing BA for sedation or muscle relaxation (for example, midazolam, lorazepam, vecuronium), for blood pressure management, or for chronic lung disease (for example, dexamethasone, hydrocortisone). The highest exposure in this study was 7.9 mg kg−1 per day which is significantly lower than the fatal doses neonates were exposed to in the 1980 s (99 to 234 mg kg−1 per day).7
The median daily PG exposure in the study population (6.3 mg kg−1 per day) was within the WHO acceptable and safe daily intakes for adults (of 0 to 25 mg kg−1 per day). However, 11% of the VLBWs who were exposed did reach levels above this threshold. The five patients with the highest PG exposure received 2.1 to 4.5 times the WHO ADI. Although a prior study suggested that exposure to 34 mg kg−1 per day of PG did not alter renal, hepatic or metabolic function in preterm infants in the short term, long term neurodevelopmental outcomes of PG exposure were not evaluated.27
The AAP strongly discourages the intake of any form of alcohol during pregnancy20 and safe exposure levels for VLBWs are unknown. Any exposure to ET in the preterm neonate may impact the developing brain and other systems. ET is found in medications commonly used during the chronic phase of NICU hospitalization in infants. A recent study showed that blood alcohol concentrations are relatively low in neonates weighing ~2000 g that were given medications containing ET.28 However some infants showed elevated levels of acetaldehyde, a potentially toxic metabolite of ET.28 In our study, higher ET exposure was associated with longer length of stay and increased odds of BPD.
The preterm population has unique developmental and physiologic factors that influence the distribution and metabolism of drugs compared with term infants, children or adults. The renal system is extremely important for drug excretion, yet the kidneys are not fully developed at birth even in a full-term neonate.29 Similarly, hepatic enzymes important for metabolism, such as cytochrome P450 and alcohol dehydrogenase, may be immature and not fully functional. One result is that preterm neonates may metabolize excipients differently, and both the half-life and the clearance rate may be longer than that of an adult..30 If toxic metabolites accumulate and diffuse across the immature blood brain barrier, adverse neurodevelopmental sequelae are possible consequences.
Both PG and ET are metabolized by alcohol dehydrogenase. Since these excipients may be administered concurrently, competitive utilization of metabolic enzymes may further impact toxic effects. In our cohort, 90 subjects (85%) received more than one excipient, and 57 (54%) subjects received both ET and PG.
In 1997, the AAP Committee on Drugs reiterated its 1985 recommended to the FDA that over-the-counter and prescription medications be labeled to include a list of inactive ingredients.12 However, prescribers in inpatient settings, including the NICU, may still be unaware of the 'inactive' ingredients contained in the medications prescribed. In the course of this study we found that accessing the excipient content of certain preparations was challenging, even when pharmaceutical companies and their marketing companies were contacted directly.
Although it would be optimal to obtain preservative- and excipient-free medications, these may not always be available and/or may be more expensive. If two medications are likely to give similar therapeutic benefits, knowing excipient content may help guide providers to choose the medication with the lowest excipient burden. It would be ideal that medications given as the standard of care in the newborn period, like vitamin K, have excipient-free options, because all newborns are expected to receive this medication unless contraindicated.
Our study was designed to define exposure to three excipients: BA, PG and ET. We obtained a complete data set of medications received and the excipient content of each medication. Our subjects were exposed to several excipients other than the excipients described here, but we chose to focus on three common excipients in neonatal medications that have been shown from prior studies to be harmful in the neonatal population. This study may underestimate excipient exposure in the NICU because only certain types of medications (PO, IV, intramuscular) were included in the analysis. In addition, owing to the retrospective nature of the study, we can only demonstrate associations between excipient exposure and outcomes, not causality. However, the levels of excipient exposure calculated are high enough to warrant concern, especially as these data reflect exposure risk even when our pediatric pharmacy has sought to obtain excipient-free medications for the NICU when feasible. Institutions who are not currently seeking excipient-free medications would likely have higher excipient exposure than seen in our study.
To our knowledge, this is the first study delineating exposure to these three excipients in the preterm VLBW population during NICU hospitalization. It will be important for future studies to prospectively assess excipient exposure and to evaluate neurodevelopmental impact of excipient exposure.
Conclusions
Preterm neonates are exposed to excipients during their NICU hospitalization, and some VLBW neonates receive more than the adult ADI. A safe level of excipient exposure has not been determined for neonates, instead these data are extrapolated from adult studies. Providers should be aware of the presence and concentration of excipients added to medications and the potential toxicities, in a similar fashion to the active component of prescribed medications. The impact of excipient exposure in VLBW infants needs further evaluation. Future studies will focus on the neurodevelopmental impact of excipient exposure in this vulnerable population.
References
Shehab N, Lewis CL, Streetman DD, Donn SM . Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med 2009; 10 (2): 256–259.
Whittaker A, Currie AE, Turner MA, Field DJ, Mulla H, Pandya HC . Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed 2009; 94 (4): F236–F240.
Souza A Jr, Santos D, Fonseca S, Medeiros M, Batista L, Turner M et al. Toxic excipients in medications for neonates in brazil. Eur J Pediatr 2014; 173 (7): 935–945.
Nellis G, Metsvaht T, Varendi H, Toompere K, Lass J, Mesek I et al. Potentially harmful excipients in neonatal medicines: a pan-european observational study. Arch Dis Child 2015; 100 (7): 694–699.
LeBel M, Ferron L, Masson M, Pichette J, Carrier C . Benzyl alcohol metabolism and elimination in neonates. Dev Pharmacol Ther 1988; 11 (6): 347–356.
McCloskey SE, Gershanik JJ, Lertora JJ, White L, George WJ . Toxicity of benzyl alcohol in adult and neonatal mice. J Pharm Sci 1986; 75 (7): 702–705.
Gershanik J, Boecler B, Ensley H, McCloskey S, George W . The gasping syndrome and benzyl alcohol poisoning. N Engl J Med 1982; 307 (22): 1384–1388.
Hiller JL, Benda GI, Rahatzad M, Allen JR, Culver DH, Carlson CV et al. Benzyl alcohol toxicity: Impact on mortality and intraventricular hemorrhage among very low birth weight infants. Pediatrics 1986; 77 (4): 500–506.
Menon PA, Thach BT, Smith CH, Landt M, Roberts JL, Hillman RE et al. Benzyl alcohol toxicity in a neonatal intensive care unit. incidence, symptomatology, and mortality. Am J Perinatol 1984; 1 (4): 288–292.
Brown WJ, Buist NR, Gipson HT, Huston RK, Kennaway NG . Fatal benzyl alcohol poisoning in a neonatal intensive care unit. Lancet 1982; 1 (8283): 1250.
MacDonald MG, Getson PR, Glasgow AM, Miller MK, Boeckx RL, Johnson EL . Propylene glycol: Increased incidence of seizures in low birth weight infants. Pediatrics 1987; 79 (4): 622–625.
American Academy of Pediatrics Committee on Drugs. "Inactive" ingredients in pharmaceutical products: Update (subject review) Pediatrics 1997; 99 (2): 268–278.
Wilson KC, Reardon C, Theodore AC, Farber HW . Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: A case series and prospective, observational pilot study. Chest 2005; 128 (3): 1674–1681.
Glasgow AM, Boeckx RL, Miller MK, MacDonald MG, August GP, Goodman SI . Hyperosmolality in small infants due to propylene glycol. Pediatrics 1983; 72 (3): 353–355.
Murch S, Costeloe K . Hyperosmolality related to propylene glycol in an infant. BMJ 1990; 301 (6748): 389.
Tran MN, Wu AH, Hill DW . Alcohol dehydrogenase and catalase content in perinatal infant and adult livers: Potential influence on neonatal alcohol metabolism. Toxicol Lett 2007; 169 (3): 245–252.
Food and Agriculture Organization of the United Nations/World Health Organization Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA). In: International Life Sciences Institute: Washington DC, USA, 1994.
Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives, Wld Hlth Org. techn. Rep. Ser., 1974, No. 539; FAO Nutrition Meetings Report Series, 1974, No. 53. Available at Http://Www.inchem.org/documents/jecfa/jecmono/v05je90.htm[Internet].; 1974.
Nair B . Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol 2001; 20 (Suppl 3): 23–50.
Williams JF, Smith VC, COMMITTEE ON SUBSTANCE ABUSE. Fetal alcohol spectrum disorders. Pediatrics 2015; 136 (5): e1395–e1406.
American Academy of Pediatrics Committee on Drugs. Ethanol in liquid preparations intended for children. Pediatrics 1984; 73 (3): 405–407.
Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ . Births: final data for 2014. Natl Vital Stat Rep 2015; 64 (12): 1–64.
Kliegman RM, Walsh MC . Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987; 17 (4): 213–288.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK . SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 2001; 138 (1): 92–100.
Dorling JS, Field DJ, Manktelow B . Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed 2005; 90 (1): F11–F16.
Lass J, Naelapaa K, Shah U, Kaar R, Varendi H, Turner MA et al. Hospitalised neonates in estonia commonly receive potentially harmful excipients. BMC Pediatr 2012; 12 (136): 2431–12-136.
Allegaert K, Vanhaesebrouck S, Kulo A, Cosaert K, Verbesselt R, Debeer A et al. Prospective assessment of short-term propylene glycol tolerance in neonates. Arch Dis Child 2010; 95 (12): 1054–1058.
Pandya HC, Mulla H, Hubbard M, Cordell RL, Monks PS, Yakkundi S et al. Essential medicines containing ethanol elevate blood acetaldehyde concentrations in neonates. Eur J Pediatr 2016; 175 (6): 841–847.
Arant BS Jr. . Postnatal development of renal function during the first year of life. Pediatr Nephrol 1987; 1 (3): 308–313.
De Cock RF, Knibbe CA, Kulo A, de Hoon J, Verbesselt R, Danhof M et al. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol 2013; 75 (1): 162–171.
Acknowledgements
We gratefully acknowledge the assistance of Dr Nakia Eldridge Pharm. D. and Dr Omayma Kish Pharm. D. who helped with determining excipient concentrations, and Dr. Elizabeth Powell PhD and Dr Rose Viscardi M.D. who gave advice throughout this study. SMM receives funding from NIH: NIAAA AA022413, AA018693, AA006916 and AA017823. CFB receives funding from NIH: NICHD HD085928, HD085061 and the Gerber Foundation. Funding organizations had no role the design of the study, collection and analysis of data or decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Akinmboni, T., Davis, N., Falck, A. et al. Excipient exposure in very low birth weight preterm neonates. J Perinatol 38, 169–174 (2018). https://doi.org/10.1038/jp.2017.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.165
This article is cited by
-
Quantitative Investigation on Exposure to Potentially Harmful Excipients by Injection Drug Administration in Children Under 2 Years of Age and Analysis of Association with Adverse Events: A Single-Center, Retrospective Observational Study
Therapeutic Innovation & Regulatory Science (2024)
-
Potentially harmful excipients in neonatal medications: a multicenter nationwide observational study in Japan
Journal of Pharmaceutical Health Care and Sciences (2021)
-
High concentrations of urinary ethanol metabolites in neonatal intensive care unit infants
Pediatric Research (2020)
-
Pharmaceutical Excipient Exposure in a Neonatal Intensive Care Unit
Indian Pediatrics (2020)
-
Urinary metabolites of volatile organic compounds of infants in the neonatal intensive care unit
Pediatric Research (2018)