Abstract
Objective:
Neurally adjusted ventilatory assist (NAVA) is a mode of mechanical ventilation that delivers ventilatory support in synchrony to the patient’s respiratory needs using NAVA level, a proportionality constant that converts the electrical activity of the diaphragm (Edi) into a peak pressure (PIP). Recent published studies suggest that neonates can control the delivered ventilatory support through neural feedback. Systematically increasing the NAVA level initially increases the PIP while maintaining a constant Edi until an inflection point or breakpoint (BrP) is reached, at which time the PIP plateaus and the Edi signal decreases. This study was performed to establish if there is a correlation of pre- and post-extubation BrP in premature neonates.
Study Design:
NAVA level was increased by 0.5 cm H2O mcV−1 every 3 min from 0.1 to 3.0 cm H2O mcV−1. PIP and Edi Peak and Minimum were recorded. Statistics: PIP and phasic Edi (Edi peak−Edi min) were averaged for each NAVA level, plotted on a graph, and the BrP was determined by visual inspection of the inflection point for PIP. The data from the studies were then combined by averaging each variable at the BrP and for each change in NAVA level above and below the BrP.
Results:
Fifteen infants were studied for paired titration studies. PIP increased until the BrP was reached and then plateaued during both the intubated and extubated titration studies. Edi decreased after the BrP was reached during the titration studies. The BrP increased when patients were extubated from NAVA to noninvasive (NIV) NAVA. As the NAVA level rose above the BrP, PIP plateaued at a higher level and Edi decreased less during the NIV NAVA titration study.
Conclusions:
Neonates demonstrated an increase in BrP, higher PIP and Edi when extubated from NAVA to NIV NAVA. This is most likely owing to the inefficiencies of NIV ventilation and suggests that neonates require a higher NAVA level when transitioning from NAVA to NIV NAVA.
Similar content being viewed by others
Introduction
Neurally adjusted ventilatory assist (NAVA) is a mode of mechanical ventilation that delivers respiratory support, in synchrony to the patient’s respiratory drive and proportional to the electrical activity of the diaphragm (Edi). The Edi is an electrical signal generated by the brainstem in patients in accordance with the breath-to-breath variation in ventilatory needs. This signal is detected by a transesophageal electrode positioned at the level of the crural diaphragm within a specialized nasogastric tube.1 The NAVA level is a proportionality constant set by the health-care provider that determines the amount of ventilatory support delivered in response to changes in the Edi. This improves the patient–ventilator synchrony in both timing and pressure of the mechanical breath, and may contribute to the prevention of lung injury.1 Recent studies suggest a breakpoint (BrP) can be identified by titration with incremental and systematic increases in the NAVA level, and is a unique value for each patient.2, 3, 4, 5 Below the BrP, when the diaphragm is not adequately unloaded, increasing the NAVA level leads to increasing peak pressures (PIPs) and tidal volumes while maintaining a high Edi. Once the BrP is reached, peak airway pressure plateaus and the Edi starts to decrease.2, 4, 5 This reflects adequate unloading of the diaphragm to the ventilator.3, 4, 6, 7, 8
A recent study in premature neonates demonstrated a distinct pattern of respiratory unloading and an identifiable BrP as the NAVA level was incrementally increased.5 Although the study evaluated neonates on either NAVA or noninvasive (NIV) NAVA, the study did not address the difference in BrP for the same patient who was intubated and then extubated. This current study’s objective was to determine the relationship in the BrP between NAVA and NIV NAVA that may aid the clinician in selecting the optimal post-extubation NAVA level.
Methods
This was a prospective, two factorial, repeated measures, case series study conducted at Promedica Toledo Children’s Hospital Neonatal Intensive Care Unit. The Institutional Review Board approved the study and parental consent was obtained. The study population included neonates who were already stabilized and ventilated on NAVA, and were ready for extubation to NIV NAVA as determined by the treating physician. All neonates were ventilated with a Servo-I ventilator (MAQUET, Solna, Sweden). Before beginning each trial, the patients’ characteristics and baseline ventilator settings were recorded.
Titration protocol
The titration protocol was designed to emulate a similar study used in a previous trial for neonates.5 The NAVA level was reduced to 0.1 cm H2O mcV−1 for 3 min. The NAVA level was then increased incrementally by 0.5 cm H2O mcV−1 every 3 min to a maximum NAVA level of 3.0 cm H2O mcV−1. After the 18 min titration study was completed, each neonate was returned to the baseline NAVA level and extubated to NIV NAVA within a few minutes using the RAM (Neotech, Valencia, CA, USA) cannula nasal interface. The neonates were placed on a NAVA level considered appropriate by the managing physician and allowed a stabilization period of ~30 min. The titration study was repeated and the neonates were returned to the baseline settings.
Titration study data were downloaded into Microsoft Excel (Microsoft Corporation; Redmond, WA, USA) for analysis. The independent variables were NAVA level and mode of ventilation (NAVA vs NIV NAVA), and the dependent variables were PIP, Edi peak and Edi min. Phasic Edi was calculated by Edi peak−Edi min and was used for analysis. All variables were averaged over each 3 min interval.
Statistical analysis
On the basis of the previous studies,5 a sample size of 15 was estimated to be adequate to show an effect. Visual inspection of the inflection point for PIP identified the BrP in each titration study as previously validated.3, 4, 5 Initially, PIP increased with increasing NAVA levels. The BrP was determined when the slope flattened and there were no longer further increases in PIP as NAVA level continued to increase. BrP pre and post extubation was compared by the paired t-test. The data from the studies were then combined by averaging each variable at the BrP and for each change in NAVA level above and below the BrP.
Results
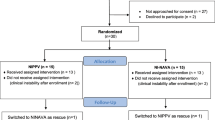
A total of 19 premature neonates were recruited and 15 patients were studied. Of the four patients not studied, one died (before reaching extubation criteria), one self-extubated before the intubated titration study could be carried out and two were electively extubated by the treating physician when the investigators were unavailable. Patient characteristics are birth weight of 950±450 g (range: 590 to 2055 g), gestational age of 26.8±2.7 weeks (range: 23 to 30 weeks) and age at study 3±2 days (range: 1 to 10 days). Reasons for delivery were combinations of preterm labor (8/15), pregnancy-induced hypertension (6/15), abruption (2/15), severe oligohydramnios (1/15), premature rupture of membranes (1/15), maternal seizure (1/15) and hemolysis, elevated liver enzymes, low platelets syndrome (1/15). A total of 74% received prenatal steroids and 53% were delivered by cesarean section. Median APGAR scores were 3 at 1 min and 7 at 5 min. Indication for mechanical ventilation was respiratory distress syndrome (14/15) and respiratory distress syndrome with pulmonary hypertension (1/15). A total of 93% received surfactant and all infants were treated with caffeine prior to extubation.
NAVA settings prior to extubation (chosen by the treating physician) were NAVA levels 1 to 1.5 cm H2O mcV−1, positive end expiratory pressure 5 to 6 cm H2O, apnea time 2 to 10 s, PIP limit 35 cm H2O and variable backup settings determined by the treating physician. NIV NAVA settings after extubation (chosen by the treating physician) were NAVA levels 1.5 to 2 cm H2O mcV−1 and positive end expiratory pressure 5 to 9 cm H2O, and the same apnea time, PIP limit and backup settings as when intubated. RAM cannula was used as the NIV interface and the positive end expiratory pressure was increased by 2 to 3 cm H2O above the desired positive end expiratory pressure level at the nasal interface to compensate for pressure loss along the RAM.9, 10 All settings, except NAVA level and FiO2, were constant throughout the trial.
A representative patient is shown in Figure 1. As NAVA level increases, PIP increases, but Edi essentially is unchanged until the BrP is reached. After the BrP is reached, PIP plateaus and Edi decreases. This is shown for both intubated (NAVA—panel A) and extubated (NIV NAVA—panel B) titration studies. In this particular patient, the BrP increased 50% from 1 on NAVA to 1.5 cm H2O mcV−1 on NIV NAVA after extubation. Edi decreased by 73% on NAVA, but by 33% on NIV NAVA, and after the BrP, both Edi and PIP were higher on NIV NAVA.
Titration studies of a representative patient on NAVA (panel a) and NIV NAVA (panel b). Both studies show the characteristic increase in peak pressure (PIP) until the breakpoint (BrP) is reached. The PIP then plateaus with no further increase despite the increasing NAVA level. Edi remains high until the BrP is reached and then decreases showing downregulation in respiratory drive with increases in the NAVA level. Edi, electrical activity of the diaphragm; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive.
Composite data for PIP for the intubated and extubated titrations normalized to BrP are shown in Figure 2. Variance was similar between both groups. BrPs were evident in all studies. Overall, the BrP increased by 33% from 1.2 cm H2O mcV−1 on NAVA to 1.6 cm H2O mcV−1 on NIV NAVA (P=0.017). The corresponding PIP at the BrP also increased by 20% (15 to 18 cm H2O).
Titration curves comparing peak inspiratory pressure (cm H2O) on NAVA and NIV NAVA. The breakpoint increased from an average of 1.2 cm H2O mcV−1 intubated on NAVA (large square) to 1.6 cm H2O mcV−1 after extubation to NIV NAVA (large triangle). The peak pressure plateau increases from 16 cm H2O on NAVA to 20 cm H2O after extubation on NIV NAVA. BrP, breakpoint; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive.
Figure 3 shows the composite data for Edi for the intubated and extubated titrations normalized to BrP. Below the BrP, Edi was constant and then decreased with increasing NAVA level above the BrP. The Edi prior to the BrP was similar between NAVA and NIV NAVA titrations, but after the BrP was reached, Edi decreased by 56% on NAVA and by 33% on NIV NAVA.
Titration curves comparing Edi (mcV) on NAVA and NIV NAVA. The breakpoint increased from an average of 1.2 cm H2O mcV−1 intubated on NAVA (large square) to 1.6 cm H2O mcV−1 after extubation to NIV NAVA (large triangle). Edi was similar in both groups below the BrP, but decreased less on NIV NAVA compared with the decrease on NAVA. BrP, breakpoint; Edi, electrical activity of the diaphragm; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive.
No adverse events occurred during catheter placement or titration studies, all vital signs (heart rate, respiratory rate, blood pressure and oxygen saturations) were stable, and specifically, there were no oxygen desaturations or bradycardia noted during any titration study.
Discussion
This is the first study that addresses changes in BrP when transitioning from NAVA to NIV NAVA. Both composite titrations demonstrated a BrP and the two-phased response to increasing NAVA levels that have been previously reported in neonates,5 and in rabbits and critically ill adults.2
This study confirms the presence of a BrP in neonates and the ability of neonates to use the feedback mechanisms in response to potential lung overdistention.5 Once the lung is adequately unloaded (at the BrP), the respiratory drive (Edi) is downregulated and there is no longer any increase in PIP despite the increase in NAVA support. It remains unclear what the contribution of these various feedback mechanisms may be. Activation of pulmonary stretch receptors by lung inflation, provides negative feedback to the respiratory center and terminates the breath (Herring–Breuer reflex), thereby preventing lung overinflation.11 Feedback from blood gases, specifically, CO2 receptors as well as the extrapulmonary mechanoreceptors sensitive to respiratory muscle load and fatigue may also contribute to this observation.3
Both the BrP and PIP on NIV NAVA were higher than on NAVA. At low levels of NAVA support, Edi was comparably high between NAVA and NIV NAVA. Although the Edi decreased after the BrP at higher NAVA levels on both NAVA and NIV NAVA, the Edi of the neonates on NIV NAVA did not decrease as much, suggesting the need to maintain a higher respiratory drive when on NIV ventilation even when over supported. It is unlikely that these increases are owing to a need for increased support, but most likely reflects the inefficiency of NIV ventilation. Considering there are significant leaks at the nasal interface, it is understandable why the neonate generates higher Edi to deliver more PIP to compensate for the PIP lost to the air leak. Several other factors may account for these increases. After extubation, NIV ventilation increases the physiologic dead space,12 because the interface between patient and ventilator shifts from the lower airway via an endotracheal tube to the upper airway using a nasal interface. Premature neonates also have a higher proportion of tracheomalacia than term neonates as a side effect of intubation and immature cartilage development.13
Visual inspection of the titrations was previously validated in both healthy and critically ill adults.2, 3, 4 A previous study in neonates also validated this method with a nonlinear regression analysis for BrP selection and found comparable BrP identification between these methods.5 At each NAVA level, 3 minute trials were considered adequate to see a response in PIP and Edi. Previous studies demonstrated that as few as 6 to 8 breaths are sufficient for changes in assist to be evident14 and that 3 min were sufficient to show a response to changes in NAVA levels.3
Previous studies with the RAM cannula have shown that the pressure needed at the ventilator is 2 to 3 cm H2O higher than other nasal interfaces when measured at the nose.9, 10 Therefore, the use of the RAM cannula as the NIV nasal interface may have influenced the absolute PIP values on NIV NAVA. In addition, large and variable air leaks at the nasal interface inherently make pressure measurements difficult to interpret.15 Although different nasal interfaces may affect the absolute PIP values observed, it is unlikely that this influenced the PIP response seen in response to the increasing NAVA levels.
Limitations of the study include the inability to determine exactly what pressure was reaching the neonates’ lungs by measuring transpulmonary pressures. Although previous studies in rabbits and adults have measured transpulmonary pressures utilizing an esophageal balloon,3, 7 this technology was not readily available for a neonatal population. Previous studies also have described a similar demonstration of BrP with tidal volume changes and increasing NAVA levels,2, 5 however, this study was unable to compare intubated versus extubated tidal volumes owing to the inability to measure accurately the delivered tidal volumes on NIV NAVA owing to the air leaks. Other limitations include the inability to extrapolate these data to other types of neonatal respiratory diseases (like chronic lung disease or pulmonary interstitial emphysema), or account for specific gestational ages and the intrinsic respiratory variability seen in premature neonates.
Conclusion
Preterm neonates ventilated invasively and noninvasively with NAVA showed differences in BrP, PIP and Edi before and after extubation. Although a higher BrP combined with higher PIP and less decrease in Edi above the BrP was evident on NIV NAVA, this most likely does not reflect the need for increased support, but rather reflects the inefficiency of NIV ventilation from variable air leaks at the nasal interface.
Clinical implications are for care providers to increase the NAVA level when extubating from NAVA to NIV NAVA, and anticipate a higher PIP to be delivered by the ventilator on NIV NAVA to overcome the large and variable air leak at the nasal interface and the resultant loss of PIP delivered to the lungs.
References
Sinderby C . Neurally adjusted ventilatory assist (NAVA). Minerva Anestesiol 2002; 68 (5): 378–380.
Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest 2009; 135 (3): 695–703.
Lecomte F, Brander L, Jalde F, Beck J, Qui H, Elie C et al. Physiological response to increasing levels of neurally adjusted ventilatory assist (NAVA). Respir Physiol Neurobiol 2009; 166 (2): 117–124.
Ververidis D, Van Gils M, Passath C, Takala J, Brander L . Identification of adequate neurally adjusted ventilatory assist (NAVA) during systematic increases in the NAVA level. IEEE Trans Biomed Eng 2011; 58 (9): 2598–2606.
Firestone KS, Fisher S, Reddy S, White DB, Stein H . Effect of changing NAVA levels on peak inspiratory pressures and electrical activity of the diaphragm in premature neonates. J Perinatol 2015; 35 (8): 612–616.
Patroniti N, Bellani G, Saccavino E, Zanella A, Grasselli G, Isgro S et al. Respiratory pattern during neurally adjusted ventilatory assist in acute respiratory failure patients. Intensive Care Med 2011; 38 (2): 230–239.
Brander L, Sinderby C, Lecomte F, Leong-Poi H, Bell D, Beck J et al. Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and non-pulmonary organ dysfunction in rabbits with acute lung injury. Intensive Care Med 2009; 35 (11): 1979–1989.
Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P et al. Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest 2007; 131 (3): 711–717.
Bailes S, Firestone KS, Dunn D, McNinch N, Brown MK, Volsko T . Evaluating the effect of flow and interface type on pressures delivered with bubble CPAP in a simulated model. Respir Care 2016; 61 (3): 333–339.
Reddy S, Fisher S, White DB, Stein H . Pilot study to compare two nasal airway interfaces on non-invasive neurally adjusted ventilatory assist. Neonatal Intensive Care 2015; 28 (1): 40–42.
Hering E, Breuer J . Die selbststeurung der athmung den nervus vagus. SitzberDeutAkadWissWein 1868; 57: 672–677.
Lumb A. Nunn’s Applied Respiratory Physiology, 6th edn. Elsevier: Philadelphia, PA, USA, 2005..
Hysinger E, Panitch H . Paediatric tracheomalacia. Paediatr Respir Rev 2016; 17: 9–15.
Vitale V, Duperret S, Mahul P, Delafosse B, Delpuech C, Weismann D et al. Time course evolution of ventilatory responses to inspiratory unloading in patients. Am J Respir Crit Care Med 1998; 157: 428–434.
Schmalisch G, Fischer H, Roehr CC, Proquitté H . Comparison of different techniques to measure air leaks during CPAP treatment in neonates. Med Eng Phys 2009; 31 (1): 124–130.
Acknowledgements
This study was supported by Promedica Toledo Children’s Hospital and Akron Children’s Hospital.
Author contributions
BL, KSF and HMS contributed to the study concept and design, data collection (BL and HMS), analysis and interpretation, and in writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HMS and KSF are speakers for MAQUET. BL declares no conflict of interest.
Rights and permissions
About this article
Cite this article
LoVerde, B., Firestone, K. & Stein, H. Comparing changing neurally adjusted ventilatory assist (NAVA) levels in intubated and recently extubated neonates. J Perinatol 36, 1097–1100 (2016). https://doi.org/10.1038/jp.2016.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.152
This article is cited by
-
Non-invasive neurally adjusted ventilatory assist in preterm infants with RDS: effect of changing NAVA levels
European Journal of Pediatrics (2022)
-
Evaluating peak inspiratory pressures and tidal volume in premature neonates on NAVA ventilation
European Journal of Pediatrics (2021)