Abstract
Objective:
Preterm infants are at risk of circulatory compromise following birth. Functional neonatal echocardiography including superior vena cava (SVC) flow is increasingly used in neonatal medicine, and low SVC flow has been associated with adverse outcome. However, echocardiography is not readily available in many neonatal units and B-type natriuretic peptides (BNPs) may be useful in guiding further cardiovascular assessment. This study investigated the relationship between BNP, N-terminal pro-BNP (NTproBNP) and echocardiographic measurements of systemic blood flow in very preterm infants.
Study Design:
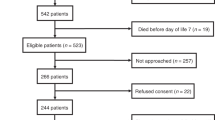
This is a prospective observational study. Sixty preterm infants <32 weeks gestational age were included after the treating neonatologist had requested an echocardiogram for suspected cardiovascular compromise. BNP and NTproBNP were sampled just before the echocardiogram. Echocardiographic examination included fractional shortening (FS), SVC flow, left and right ventricular output (LVO and RVO). Statistical analysis included simple linear regression of BNP and NTproBNP with echocardiographic measures and multiple regression including potential confounding variables.
Result:
Mean (s.d.) gestational age at birth was 275 (21) weeks, median (interquartile range, IQR) birth weight was 995 (845 to 1175) grams. Neither BNP nor NTproBNP correlated with SVC flow (BNP 95% confidence interval (CI) −0.0014 to 0.013, P=0.12; NTproBNP 95% CI −0.00069 to 0.01, P=0.085); LVO (BNP 95% CI −0.00078 to 0.0072, P=0.11; NTproBNP 95% CI −0.0034 to 0.0034, P=0.99); RVO (BNP 95% CI −0.00066 to 0.0058, P=0.12; NTproBNP 95% CI −0.0012 to 0.0044, P=0.25); or FS (BNP 95% CI −0.053 to 0.051, P=0.96; NTproBNP 95% CI −0.061 to 0.019, P=0.3). Multivariate linear regression did not significantly alter results.
Conclusion:
In this cohort of very preterm infants, BNP and NTproBNP did not correlate with echocardiographic measurements of systemic blood flow within the first 72 h of life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kluckow M, Seri I, Evans N . Functional echocardiography: an emerging clinical tool for the neonatologist. J Pediatr 2007; 150: 125–130.
Evans N, Gournay V, Cabanas F, Kluckow M, Leone T, Groves A et al. Point-of-care ultrasound in the neonatal intensive care unit: international perspectives. Semin Fetal Neonatal Med 2011; 16: 61–68.
El-Khuffash AF, McNamara PJ . Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin Fetal Neonatal Med 2011; 16: 50–60.
Kluckow M, Evans N . Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 2000; 82: F182–F187.
Kluckow M, Evans N . Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 2000; 82: F188–F194.
Hunt RW, Evans N, Rieger I, Kluckow M . Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 2004; 145: 588–592.
Evans N, Kluckow M . Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 1996; 74: F88–F94.
Daniels LB, Maisel AS . Natriuretic peptides. J Am Coll Cardiol 2007; 50: 2357–2368.
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347: 161–167.
Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P et al. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol 2003; 41: 2010–2017.
Januzzi Jr. JL, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95: 948–954.
Law YM, Hoyer AW, Reller MD, Silberbach M . Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: the Better Not Pout Children! Study. J Am Coll Cardiol 2009; 54: 1467–1475.
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001; 345: 1014–1021.
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003; 107: 2786–2792.
Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002; 105: 2392–2397.
Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004; 350: 655–663.
Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M . Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 1943–1950.
Hutfless R, Kazanegra R, Madani M, Bhalla MA, Tulua-Tata A, Chen A et al. Utility of B-type natriuretic peptide in predicting postoperative complications and outcomes in patients undergoing heart surgery. J Am Coll Cardiol 2004; 43: 1873–1879.
Davlouros PA, Karatza AA, Xanthopoulou I, Dimitriou G, Georgiopoulou A, Mantagos S et al. Diagnostic role of plasma BNP levels in neonates with signs of congenital heart disease. Int J Cardiol 2011; 147: 42–46.
Reynolds EW, Ellington JG, Vranicar M, Bada HS . Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics 2004; 114: 1297–1304.
Baptista MJ, Rocha G, Clemente F, Azevedo LF, Tibboel D, Leite-Moreira AF et al. N-terminal-pro-B type natriuretic peptide as a useful tool to evaluate pulmonary hypertension and cardiac function in CDH infants. Neonatology 2008; 94: 22–30.
Holmstrom H, Hall C, Thaulow E . Plasma levels of natriuretic peptides and hemodynamic assessment of patent ductus arteriosus in preterm infants. Acta Paediatr 2001; 90: 184–191.
Puddy VF, Amirmansour C, Williams AF, Singer DR . Plasma brain natriuretic peptide as a predictor of haemodynamically significant patent ductus arteriosus in preterm infants. Clin Sci (Lond) 2002; 103: 75–77.
Flynn PA, da Graca RL, Auld PA, Nesin M, Kleinman CS . The use of a bedside assay for plasma B-type natriuretic peptide as a biomarker in the management of patent ductus arteriosus in premature neonates. J Pediatr 2005; 147: 38–42.
Sanjeev S, Pettersen M, Lua J, Thomas R, Shankaran S, L’Ecuyer T . Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J Perinatol 2005; 25: 709–713.
El-Khuffash AF, Amoruso M, Culliton M, Molloy EJ . N-terminal pro-B-type natriuretic peptide as a marker of ductal haemodynamic significance in preterm infants: a prospective observational study. Arch Dis Child Fetal Neonatal Ed 2007; 92: F421–F422.
Czernik C, Lemmer J, Metze B, Koehne PS, Mueller C, Obladen M . B-type natriuretic peptide to predict ductus intervention in infants <28 weeks. Pediatr Res 2008; 64: 286–290.
Ramakrishnan S, Heung YM, Round J, Morris TP, Collinson P, Williams AF . Early N-terminal pro-brain natriuretic peptide measurements predict clinically significant ductus arteriosus in preterm infants. Acta Paediatr 2009; 98: 1254–1259.
Attridge JT, Kaufman DA, Lim DS . B-type natriuretic peptide concentrations to guide treatment of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 2009; 94: F178–F182.
Nuntnarumit P, Khositseth A, Thanomsingh P . N-terminal probrain natriuretic peptide and patent ductus arteriosus in preterm infants. J Perinatol 2009; 29: 137–142.
Chen S, Tacy T, Clyman R . How useful are B-type natriuretic peptide measurements for monitoring changes in patent ductus arteriosus shunt magnitude? J Perinatol 2010; 30: 780–785.
Hsu JH, Yang SN, Chen HL, Tseng HI, Dai ZK, Wu JR . B-type natriuretic peptide predicts responses to indomethacin in premature neonates with patent ductus arteriosus. J Pediatr 2010; 157: 79–84.
Letzner J, Berger F, Schwabe S, Benzing J, Morgenthaler NG, Bucher HU et al. Plasma C-terminal pro-endothelin-1 and the natriuretic pro-peptides NT-proBNP and MR-proANP in very preterm infants with patent ductus arteriosus. Neonatology 2012; 101: 116–124.
Joseph L, Nir A, Hammerman C, Goldberg S, Ben Shalom E, Picard E . N-terminal pro-B-type natriuretic peptide as a marker of bronchopulmonary dysplasia in premature infants. Am J Perinatol 2010; 27: 381–386.
Czernik C, Metze B, Muller C, Muller B, Buhrer C . Urinary N-terminal B-type natriuretic peptide predicts severe retinopathy of prematurity. Pediatrics 2011; 128: e545–e549.
Sahn DJ, DeMaria A, Kisslo J, Weyman A . Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083.
Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M . Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr 2012; 160: 943–948.
Cantinotti M, Storti S, Parri MS, Prontera C, Murzi B, Clerico A . Reference intervals for brain natriuretic peptide in healthy newborns and infants measured with an automated immunoassay platform. Clin Chem Lab Med 2010; 48: 697–700.
da Graca RL, Hassinger DC, Flynn PA, Sison CP, Nesin M, Auld PA . Longitudinal changes of brain-type natriuretic peptide in preterm neonates. Pediatrics 2006; 117: 2183–2189.
Mannarino S, Garofoli F, Cerbo RM, Perotti G, Mongini E, Codazzi C et al. Cord blood, perinatal BNP values in term and preterm newborns. Arch Dis Child Fetal Neonatal Ed 2010; 95: F74.
Mir TS, Laux R, Hellwege HH, Liedke B, Heinze C, von Buelow H et al. Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth. Pediatrics 2003; 112: 896–899.
Farombi-Oghuvbu I, Matthews T, Mayne PD, Guerin H, Corcoran JD . N-terminal pro-B-type natriuretic peptide: a measure of significant patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 2008; 93: F257–F260.
El-Khuffash A, Davis PG, Walsh K, Molloy EJ . Cardiac troponin T and N-terminal-pro-B type natriuretic peptide reflect myocardial function in preterm infants. J Perinatol 2008; 28: 482–486.
Mannarino S, Garofoli F, Mongini E, Cerbo RM, Codazzi AC, Tzialla C et al. BNP concentrations and cardiovascular adaptation in preterm and fullterm newborn infants. Early Hum Dev 2010; 86: 295–298.
Osborn DA, Evans N, Kluckow M . Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch Dis Child Fetal Neonatal Ed 2004; 89: F168–F173.
Miletin J, Dempsey EM . Low superior vena cava flow on day 1 and adverse outcome in the very low birthweight infant. Arch Dis Child Fetal Neonatal Ed 2008; 93: F368–F371.
Buddhe S, Dhuper S, Kim R, Weichbrod L, Mahdi E, Shah N et al. NT-proBNP levels inprove the ability of predicting a hemodynamically significant patent ductus arteriosus in very low-birth-weight infants. J Clin Neonatol 2012; 1: 82–86.
Acknowledgements
We would like to thank all the families for their participation in this trial, all NICU nurses and Dr Clare Collins, Mercy Hospital for Women, and Jennifer Horvath (Austin Pathology, Melbourne, Australia) for their support. We gratefully acknowledge The Medical Research Foundation for Women and Babies, East Melbourne, Australia, for supporting this study. The Foundation had no involvement in study design, collection, analysis, and interpretation of data, writing and submission of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
König, K., Guy, K., Walsh, G. et al. The relationship between BNP, NTproBNP and echocardiographic measurements of systemic blood flow in very preterm infants. J Perinatol 34, 296–300 (2014). https://doi.org/10.1038/jp.2014.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.2
Keywords
This article is cited by
-
NTproBNP is a useful early biomarker of bronchopulmonary dysplasia in very low birth weight infants
European Journal of Pediatrics (2019)
-
Factors affecting N-terminal pro-B-type natriuretic peptide levels in preterm infants and use in determination of haemodynamic significance of patent ductus arteriosus
European Journal of Pediatrics (2018)