Abstract
Objective:
We hypothesize that a complete blood count (CBC) with manual differential from umbilical cord blood is equivalent to a CBC with manual differential obtained from the neonate on admission.
Study Design:
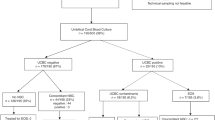
A CBC and manual differential was performed on 174 paired umbilical cord blood and admission blood samples from infants <35 weeks gestation. Paired t-test and Pearson's correlation coefficient were the primary statistical tools used for data analysis.
Result:
Cord and admission blood white blood cell (WBC) count, hemoglobin and platelet count all significantly (P<0.0001) correlated with paired neonatal samples (R=0.82, 0.72, 0.76). Admission blood WBC count fell within the variation of WBC count values from currently accepted neonatal admission blood sources. Cord blood hemoglobin was not clinically different than admission hemoglobin (1.0 g dl−1). Cord blood platelet counts were not different from admission blood platelet counts (5800 cells per μl, P=0.23). The immature to total granulocyte ratio was not different between samples (P=0.34).
Conclusion:
Umbilical cord blood can be used for admission CBC and differential in premature infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hack M, Fanaroff AA . Outcomes of children of extremely low birth weight and gestational age in the 1990s. Semin Neonatol 2000; 5: 89–106.
Lemons AJ, Bauer CR, Oh W, Koreones SB, Papile LA, Stoll BJ . Very low birth weight outcomes of the National Institute of Health and Human Development National Researach Network, January 1995 through December 1996. Pediatrics 1996; 107: el.
Serenius F, Ewald U, Farooqi A, Holmgren P . Short-term outcome after active perinatal management at 23–24 weeks of gestation. A study from two Swedish tertiary care centres. Part 2: infant survival. Acta Paediatrica 2004; 93: 1081–1089.
Alandangady N, McHugh S, Aitchison TC, Wardrop CAJ, Holland BM . Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics 2006; 117: 93–98.
Saigal S, O’Neill A, Surainder Y, Chua LB, Usher R . Placental transfusion and hyperbilirubinemia in the premature. Pediatrics 1972; 49: 406–419.
Usher R, Lind J . Blood volume of the newborn premature Infant. Acta Paediatrica Scandinavica 1965; 54 (Sept.): 419–431.
Vital Stats. National Vital Statistics System http://205.207.175.93/VitalStats/TableViewer/tableView.aspx?ReportId=15097Accessed November 23, 2009.
Hansen A, Forbes P, Buck R . Potential substitution of cord blood for infant blood in the neonatal sepsis evaluation. Biol Neonate 2005; 88: 12–18.
Costakos DT, Walden J, Rinzel MT, Dahlen L . Painless blood testing to prevent neonatal sepsis. WMJ Sep 2009; 108 (6): 321–322.
Dahlbach B . Blood Coagulation. Lancet 2000; 355: 1627–1632.
Bain BJ . Blood Cells, A Practical Guide. 3rd edn. Blackwell Publishing, 2003.
Walka MM, Sonntag J, Kage A, Dudenhausen JW, Obladen M . Complete blood counts from umbilical cords of healthy term newborns by two automated cytometers. Acta Haematologica. 1998; 105: 71–76.
Lee HR, Shin S, Yoon JH et al. Complete blood count reference values of donated cord blood from Korean neonates. Korean J Lab Med. 2009; 29: 179–184.
Jansen M, Brand A, von Lindern JS, Scherjon S, Walther FJ . Potential use of autologous umbilical cord blood red blood cells for early transfustion needs of premature infants. Transfusion 2006; 46: 1049–1056.
Rabe H, Wacker A, Hulskamp G et al. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. Eur J Pediatr 2000; 159: 775–777.
Hosono S, Mugishima H, Fujita H et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks gestation: a randomised controlled trial. 2007; 93: F14–F19.
Hosono S, Mugishima H, Fujita H et al. Blood pressure and urine output during the first 120 h of life in infants born at less than 29 weeks gestation related to umbilical cord milking. 2009; 94: F328–F331.
Rabe H, Reynolds G, Diaz-Rossello J . A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology 2008; 93: 138–144.
Manroe BL, Weinberg AG, Rosenfeld CR, Browne R . The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979; 95 (1): 89–98.
Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R . Revised reference ranges for circulating neutrophils in very low birth weight neonates. Pediatrics 1994; 76–82.
Christensen RD, Rothstein G . Pitfalls in the interpretation of leukocyte counts of newborn infants. Am J Clin pathol 1979; 72: 608–611.
Thurlbeck SM, McIntosh N . Preterm blood counts vary with sampling site. 1987; 62: 74.
Moe PJ . Umbilical cord blood and capillary blood in the evaluation of anemia in erythroblastosis fetalis. Acta Paediatrica Scandinavica. 1967; 56: 391.
Linderkamp O, Versmold HT, Strohhacker I . Capillary-venous hematocrit differences in newborn infants. Eur J Pediatr 1977; 127: 9.
Christensen RD . Expected Hematologic Values for Term and Preterm Neonates. Hematologic Problems of the Neonate. Philadelphia, PA: WB Saunders, 2000; 117–136.
Oh W, Lind J . Venous and capillary hematocrit in newborn infants and placental transfusion. Acta Paediatrica Scandinavica 1966; 38–48.
Burman D, Morris A . Cord haemoglobin in low birth weight infants. Arch Dis Child. 1974; 46: 382–385.
Rooth G, Sjostedt S . Haemoglobin in cord blood in normal and prolonged pregnancy. Arch Dis Child. 1957; 93: 486–487.
Jopling J, Henry E, Wiedmeier SE, Christensen RD . Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: Data from a multihospital health care system. Pediatrics 2009; 123 (2): e333–e337.
Foley ME, Clayton JK, McNicol GP . Haemostatic mechanisms in maternal, umbilical vein and umbilical artery blood at the time of delivery. Br J Obstetr Gynaecol 1977; 84: 81–87.
Pedersen NT, Petersen S . Megakaryocytes in the foetal circulation and in cubital venous blood in the mother before and after delivery. Scandinavian J Haematol 1980; 25: 5–11.
Acknowledgements
We would like to acknowledge the obstetric nursing staff of The Ohio State University Medical Center, for umbilical cord blood collection, Philip Binkley, MD, MPH, Courtney Lynch, PhD, MPH and Juli Richter, MD, PharmD for reviewing the manuscript before submission. This study was funded through an intramural grant provided by Nationwide Children's Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Carroll, P., Nankervis, C., Iams, J. et al. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J Perinatol 32, 97–102 (2012). https://doi.org/10.1038/jp.2011.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.60
Keywords
This article is cited by
-
Can cord blood sampling delay the first packed red blood cell transfusion?
Journal of Perinatology (2021)
-
Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study
BMC Pediatrics (2020)
-
Cord blood neutropenia is an independent predictor of early sepsis
Journal of Perinatology (2017)
-
Predictive factors and practice trends in red blood cell transfusions for very-low-birth-weight infants
Pediatric Research (2016)
-
New and underutilized uses of umbilical cord blood in neonatal care
Maternal Health, Neonatology and Perinatology (2015)