Abstract
Objective:
The objective of this trial was to test whether probiotic-supplemented feeding to extremely low-birth-weight (ELBW) infants will improve growth as determined by decreasing the percentage of infants with weight below the 10th percentile at 34 weeks postmenstrual age (PMA). Other important outcome measures, such as improving feeding tolerance determined by tolerating larger volume of feeding per day and reducing antimicrobial treatment days during the first 28 days from the initiation of feeding supplementation were also evaluated.
Study Design:
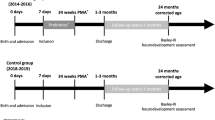
We conducted a multicenter randomized controlled double-blinded clinical study. The probiotics-supplementation (PS) group received Lactobacillus rhamnosus GG and Bifidobacterium infantis added to the first enteral feeding and continued once daily with feedings thereafter until discharge or until 34 weeks (PMA). The control (C) group received unsupplemented feedings. Infant weight and feeding volumes were recorded daily during the first 28 days of study period. Weights were also recorded at 34 weeks PMA.
Result:
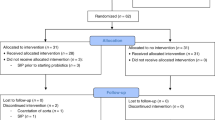
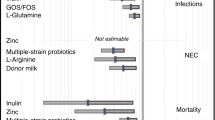
A total of 101 infants were enrolled (PS 50 versus C 51). There was no difference between the two groups in the percentage of infants with weight below the 10th percentile at 34 weeks PMA (PS group 58% versus C group 60%, (P value 0.83)) or in the average volume of feeding during 28 days after study entry (PS group 59 ml kg−1 versus C group 71 ml kg−1, (P value 0.11)). Calculated growth velocity was higher in the PS group compared with the C group (14.9 versus 12.6 g per day, (P value 0.05)). Incidences of necrotizing enterocolitis (NEC), as well as mortality were similar between the two groups.
Conclusion:
Although probiotic-supplemented feedings improve growth velocity in ELBW infants, there was no improvement in the percentage of infants with growth delay at 34 weeks PMA. There were no probiotic-related adverse events reported.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M et al. Trends in mortality and morbidity for very low birth weight infants 1991–1999. Pediatrics 2002; 110: 143–151.
Morley R, Lucas A . Influence of early diet on outcome in preterm infants. Acta Paediatr Suppl 1994; 405: 123–126.
Franz AR, Pohlandt F, Bode H, Mihatsch WA . Intrauterine, earlynonatal, and postdischarge growth neurodevelopmental outcome at 5.4 years in extremely preterm infants after intenstive neonatal nutritional support. Pediatrics 2009; 123: e101–e109.
Millar M, Wilks M, Costeloe K . Probiotics for preterm infants. Arch Dis Child Fetal Neonatal Ed 2003; 88: F354–F358.
Martin CR, Walker WA . Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol 2008; 32: 127–137.
Caplan M, Tamas J . Neonatal Necrotizing Enterocolitis: Possible role of probiotic supplementation. J pediatr Gastroenterol Nutr 2000; 30 (2): S18–S22.
Mack DR, Michail S, Wei S . Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 1999; 276: G950.
Mohan R, Koebnick C, Schildt J . Effect of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm neonates: a double placebo controlled, randomized study. J Clin Microbiol 2006; 44: 4025–4031.
Deplancke B, Gaskins HR . Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 2001; 73: 1131S–1141S.
Madsen K, Cornish A, Soper P . Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001; 121: 580–591.
Kennedy RJ, Kirk SJ, Gardiner KR . Mucosal barrier function and the commensal flora. Gut 2002; 50: 441–442.
Orrhage K, Nord CE . Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr 1999; (Suppl 88): 47–57.
Panigrahi P, Gupta S, Gewolb IH . Occurrence of necrotizing enterocolitis may be dependent on patterns of bacterial adherence and intestinal colonization: studies in Caco-2 tissue culture and weanling rabbit models. Pediatr Res 1994; 36: 115–121.
Sudo N, Sawamura S, Tanaka K . The requirement of intestinal bacterial flora for the development of IgE production system fully susceptible to oral tolerance induction. J Immunol 1997; 159: 1739–1745.
Fukushima Y, Kawata Y, Hara H . Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 1998; 42: 39–44.
Schiffrin EJ, Rochat F, Link-Amster H . Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci 1995; 78: 491–497.
Weng M, Walker WA, Sanderson IR . Butyrate regulates the expression of pathogen-triggered IL-8 in intestinal epithelia. Pediatr Res 2007; 62: 542–546.
Viljanen M, Kuitunen M, Haahtela T . Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol 2005; 16: 65–71.
Marin ML, Tejada-Simon MV, Lee JH . Stimulation of cytokine production in clonal macrophages and T-cell models by Streptococcus thermophilus: comparison with Bifidobacterium sp. and Lactobaillus bulgarius. J Food Prot 1998; 61: 859–864.
Murch SH . Toll of allergy reduced by probiotics. Lancet 2001; 357: 1057–1059.
Klinman DM, Yi AK, Beaucage SL . CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and interferon gamma. Proc Natl Acad Sci USA 1996; 93: 2879–2883.
Fujii T, Ohtsuka Y, Lee T . Bifidobacterium breve enhances transforming growth factor betal signaling by regulating Smad7 expression in preterm infants. J Pediatr gastroenterol Nutr 2006; 43: 83–88.
Takeda K, Suzuki T, Shimada SI . Interleukin-12 involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol 2006; 146: 109–115.
Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M . Early administration of Bifidobacterium breve to preterm infants: randomized controlled trial. Arch Dis Child Fetal Neonatal Ed 1997; 76: f101–f107.
Rouge C, Piloquet H, Butel M, Rochat F, Ferraris L . Oral supplementation with probiotics in very low birth weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J clin Nutr 2009; 89: 1828–1835.
Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli F . Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. Biol Neonate 2002; 82: 103–108.
Costalos C, Skouteri V, Gounaris A . Enteral feeding of premature neonates with Saccharomyces boulardii. Early Hum Dev 2003; 74: 89–96.
Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005; 147: 192–196.
Lin HC, Su BH, Chen AC . Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115: 1–4.
Manzoni P, Mostert M, Leonessa ML . Oral supplementation with Lactobacillus casei subspecies in preterm neonates: a randomized study. Clin Infect Dis 2006; 42: 1735–1742.
Startiki Z, Costalos C, Sevastiadou S . The effect of bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007; 83: 575–579.
Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI et al. Oral robiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter randomized controlled trial. Pediatrics 2008; 122: 693–700.
Samanta M, Sarkar M, Ghosh P . Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009; 55 (2): 128–131.
Deshpande G, Rao S, Patole S, Bulsara M . Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010; 125 (5): 921–930.
Soll R . Probiotic: are we ready for routine use? Pediatrics 2010; 125 (5): 1071–1072.
Patel A, Engstrom J, Meier P, Kimura R . Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics 2005; 116 (6): 1466–1473.
Kunz AN, Fairchik MP, Noel JM . Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005; 115 (1): 178–181.
Sullivan A, Nord CE . Probiotic lactobacilli and bacteraemia in Stockholm. Scand J Infect Dis 2006; 38 (5): 327–331.
Presterl E, Kneifel W, Mayer HK, Zehetgruber M, makristathis A, Graninger W . Endocarditis by Lactobacillus rhamnosus due to yogurt ingestion? Scand J Infect Dis 2001; 33 (9): 710–714.
Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Peds 2010; 156 (4): 679–681.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Al-Hosni, M., Duenas, M., Hawk, M. et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 32, 253–259 (2012). https://doi.org/10.1038/jp.2011.51
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.51
Keywords
This article is cited by
-
Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—a systematic review and meta-analysis
European Journal of Clinical Nutrition (2023)
-
Bifidobacterium infantis as a probiotic in preterm infants: a systematic review and meta-analysis
Pediatric Research (2023)
-
Bifidobacterium and Lactobacillus for preventing necrotizing enterocolitis in very-low-birth-weight preterm infants: a systematic review and meta-analysis
World Journal of Pediatrics (2020)
-
Probiotic strategies to prevent necrotizing enterocolitis in preterm infants: a meta-analysis
Pediatric Surgery International (2019)
-
Probiotic supplementation in preterm infants does not affect the risk of retinopathy of prematurity: a meta-analysis of randomized controlled trials
Scientific Reports (2017)