Abstract
Objective:
Comparison of the differences between availability of animal-derived surfactant preparations used to treat premature infants is incomplete. The objective of this study was to assess the short-term treatment efficacy of the two most commonly used surfactant preparations in the United States, beractant (100 mg kg–1 initial and subsequent doses) and poractant alfa (200 mg kg–1 initial and 100 mg kg–1 subsequent doses), in very premature, mechanically ventilated infants <30 weeks gestation with respiratory distress syndrome (RDS).
Study Design:
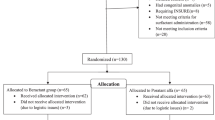
Inborn infants at two institutions, open label, 1:1, randomized controlled trial. Level of respiratory support for first 72 h of life. Morbidities of prematurity observed during the neonatal intensive care unit hospitalization.
Result:
We studied 52 infants 24 0/7 to 29 6/7 weeks gestation; 25 received poractant alfa (27.1±1.6 weeks, birth weight of 930±231 g) and 27 received beractant (26.7±1.7 weeks, P=0.343 and birth weight 900±271 g, P=0.668). Respiratory support for the first 72 h of life was lower in the poractant alfa than beractant group for mean airway pressure (MAP, P=0.003) and respiratory index (MAP × FiO2, P=0.032). Infants in the poractant alfa group had a greater number of infants extubated at 48 (13/25 vs 6/27, P=0.027) and 72 h (15/25 vs 8/27, P=0.029) than the beractant group. Although the study was not powered to detect morbidities of prematurity, the prevalence of PDA and air leaks was less in the infants treated with poractant alfa than in those treated with beractant. Rates of bronchopulmonary dysplasia (8/23 vs 11/22, P=0.303) or death (2/15 vs 5/27, P=0.272) were similar in the infants treated with poractant alfa and beractant, respectively.
Conclusion:
This study suggests significant short-term benefits to the use of the larger initial dose of poractant alfa than beractant in very premature infants with RDS. Further studies involving a larger number of preterm infants are needed to assess long-term effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Whitsett JA, Pryhuber GS, Rice WR, Warner BB, Wert SE . Acute respiratory disorders. In: Neonatology: Pathophysiology and Management of the Newborn. Avery GB, Fletcher MA, Macdonald MG (eds). Lippincott: Williams and Wilkins, Philadelphia, 1999, pp 485–508.
Martin JA, Hamilton BE, Sutton PD, Menacker F, Munson ML . Births: final data for 2002. National center for health statistics. Vital Health Stat 2003; 52: 1.
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ et al. Very low birth weight infant outcomes of the NICHD neonatal research network. Pediatrics 2001; 107: 1–8.
Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics 1997; 100: 31–38.
Moya F, Maturana A . Animal-derived surfactants versus past and current synthetic surfactants: current status. Clin Perinatol 2007; 34: 145–177.
Speer CP, Gefeller O, Groneck P, Laufkötter E, Roll C, Hanssler L et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child 1995; 72: F8–13.
Baroutis G, Kaleyias J, Liarou T, Papathoma E, Hatzistamatiou Z, Costalos C . Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur J Pediatr 2003; 162: 476–480.
Ramanathan R, Rasmussen MR, Gerstmann D, Finer N, Sekar K, North American Study Group. A randomized, multicenter masked comparison trial of Poractant and Survanta in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 2004; 21: 109–119.
Malloy CA, Nicoski P, Muraskas JM . A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr 2005; 94: 779–784.
Fujii AM, Allen R, Doros G, O’Brien S . Patent ductus arteriosus hemodynamics in very premature infants treated with poractant alfa or beractant for respiratory distress syndrome. J Perinatol (in press).
Young TE, Mangum B . NeoFAX. 16th edn 2003. Acorn Publishing, Inc.: Raleigh, NC
Pillow JJ, Neil H, Wilkinson MH, Ramsden CA . Effect of I/E ratio on mean alveolar pressure during high-frequency oscillatory ventilation. J Appl Physiol 1999; 87: 407–414.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK, for the Canadian NICU Network, The Kaiser Permanente National Minimum Data Set Wide Area Network and the SNAP-II Study Group. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 2001; 138: 92–100.
Speer CP, Robertson B, Curstedt T, Halliday HL, Compagnone D, Geffeller O et al. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics 1992; 89: 13–20.
Hammoud M, Tahlib L . Development of chronic lung disease in preterm infants treated with surfactant. Pediatr Int 2002; 44: 493–499.
Van Marter LJ, Pagano M, Allred EN, Leviton A, Kuban KC . Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr 1992; 120: 938–946.
Chong E, Greenspan J, Kirkby S, Culthane J, Dysart K . Changing use of surfactant over 6 years and its relationship to chronic lung disease. Pediatrics 2008; 122: e917–e921.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Fujii was supported by an unrestricted research grant from Dey LP, Napa, CA and Chiesi Farmaceutici Spa, Parma, Italy. The remaining authors declare no conflict of interest. The funders had no involvement in study design, in data collection, analysis/interpretation of the data, in the writing of the paper or in the decision to submit the paper for publication.
Rights and permissions
About this article
Cite this article
Fujii, A., Patel, S., Allen, R. et al. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol 30, 665–670 (2010). https://doi.org/10.1038/jp.2010.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2010.20
Keywords
This article is cited by
-
Comparative efficacy and safety of late surfactant preparations: a retrospective study
Journal of Perinatology (2021)
-
Porcine versus bovine surfactant therapy for RDS in preterm neonates: pragmatic meta-analysis and review of physiopathological plausibility of the effects on extra-pulmonary outcomes
Respiratory Research (2020)
-
Beractant and poractant alfa in premature neonates with respiratory distress syndrome: a systematic review of real-world evidence studies and randomized controlled trials
Journal of Perinatology (2020)
-
Porcine vs bovine surfactant therapy for preterm neonates with RDS: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes
Respiratory Research (2019)
-
Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future
Pediatric Research (2017)