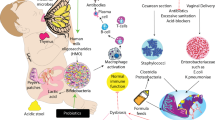
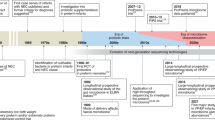
Abstract
The intestinal microbiota normally exists in a commensal and/or symbiotic relationship with the host. In the past few years, emerging technologies derived largely from the Human Genome Project have been applied to evaluating the intestinal microbiota and new discoveries using these techniques have prompted new initiatives such as the Human Microbiome Roadmap designed to evaluate the role of the intestinal microbiome in health and disease. In this review, we wish to focus on some new developments in this area and discuss some of the effects of medical manipulations such as antibiotics, probiotics, prebiotics and C-section versus vaginal delivery on the intestinal microbiota.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hooper LV, Gordon JI . Commensal host-bacterial relationships in the gut. Science 2001; 292: 1115–1118.
Macpherson AJ, Harris NL . Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 2004; 4: 478–485.
Forsythe P, Bienenstock J . Immunomodulation by commensal and probiotic bacteria. Immunol Invest 2010; 39: 429–448.
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI . The human microbiome project. Nature 2007; 449: 804–810.
Neu J, Mshvildadze M, Mai V . A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep 2008; 10: 450–457.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118: 511–521.
Orrhage K, Nord CE . Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl 1999; 88: 47–57.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010; 107: 11971–11975.
Round JL, Mazmanian SK . The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009; 9: 313–323.
Ismail AS, Hooper LV . Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 2005; 289: G779–G784.
Stappenbeck TS, Hooper LV, Gordon JI . Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA 2002; 99: 15451–15455.
Hooijkaas H, Benner R, Pleasants JR, Wostmann BS . Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered ‘antigen-free’ diet. Eur J Immunol 1984; 14: 1127–1130.
Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456: 507–510.
Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P . Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J Immunol 2003; 170: 816–822.
Strachan DP . Hay fever, hygiene, and household size. BMJ 1989; 299: 1259–1260.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010; 5: e9085.
De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol 2010; 10: 63.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484.
Holt PG, Rowe J, Loh R, Sly PD . Developmental factors associated with risk for atopic disease: implications for vaccine strategies in early childhood. Vaccine 2003; 21: 3432–3435.
Lundin A, Bok CM, Aronsson L, Björkholm B, Gustafsson JA, Pott S et al. Gut flora, Toll-like receptors and nuclear receptors: a tripartite communication that tunes innate immunity in large intestine. Cell Microbiol 2008; 10: 1093–1103.
Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y . The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997; 159: 1739–1745.
Rook GA . 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol 2010; 160: 70–79.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R . Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004; 118: 229–241.
Neish AS . Microbes in gastrointestinal health and disease. Gastroenterology 2009; 136: 65–80.
Sharma R, Young C, Neu J . Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol 2010; 2010: 305879.
Round JL, O’Connell RM, Mazmanian SK . Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun 2010; 34: J220–J225.
Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G . Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr 2008; 138: 1796S–1800S.
Gronlund MM, Lehtonen OP, Eerola E, Kero P . Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 1999; 28: 19–25.
Salminen S, Gibson GR, McCartney AL, Isolauri E . Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 2004; 53: 1388–1389.
Björkstén B . Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol 2004; 25: 257–270.
Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol 2004; 15: 48–54.
Debley JS, Smith JM, Redding GJ, Critchlow CW . Childhood asthma hospitalization risk after cesarean delivery in former term and premature infants. Ann Allergy Asthma Immunol 2005; 94: 228–233.
Laubereau B, Filipiak-Pittroff B, von Berg A, Grübl A, Reinhardt D, Wichmann HE et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch Dis Child 2004; 89: 993–997.
Eggesbø M, Botten G, Stigum H, Nafstad P, Magnus P . Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol 2003; 112: 420–426.
Jernberg C, Lofmark S, Edlund C, Jansson JK . Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007; 1: 56–66.
Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L . Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010; 5: e9836.
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO . Development of the human infant intestinal microbiota. PLoS Biol 2007; 5: e177.
DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS ONE 2008; 3: 33056.
Mshvildadze M, Neu J, Mai V . Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutr Rev 2008; 66: 658–663.
Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V . Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr 2010; 156: 20–25.
Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 2009; 3: 944–954.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ et al. NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123 (1): 58–66.
Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005; 147: 192–196.
Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122: 693–700.
Deshpande G, Rao S, Patole S, Bulsara M . Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010; 125: 921–930.
Szajewska H, Setty M, Mrukowicz J, Guandalini S . Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr 2006; 42: 454–475.
Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007; 335: 80.
Szajewska H, Ruszczynski M, Radzikowski A . Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr 2006; 149: 367–372.
Gibson GR . Dietary modulation of the human gut microflora using prebiotics. Br J Nutr 1998; 80: S209–S212.
Sherman PM, Cabana M, Gibson GR, Koletzko BV, Neu J, Veereman-Wauters G et al. Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27–28, 2008. J Pediatr 2009; 155: S61–S70.
Donovan SM . Human milk oligosaccharides—the plot thickens. Br J Nutr 2009; 101: 1267–1269.
Zhang L, Li N, Caicedo R, Neu J . Alive and dead lactobacillus rhamnosus GG decrease tumor necrosis factor-{alpha}-induced interleukin-8 production in Caco-2 cells. J Nutr 2005; 135: 1752–1756.
Zhang L, Li N, des Robert C, Fang M, Liboni K, McMahon R et al. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr 2006; 42: 545–552.
Li N, Russell WM, Douglas-escobar M, Hauser N, Lopez M, Neu J . Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res 2009; 66: 203–207.
Lopez M, Li N, Kataria J, Russell M, Neu J . Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. JNutr 2008; 138: 2264–2268.
Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 2006; 177: 3273–3282.
Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007; 179: 4808–4820.
Hamady M, Knight R . Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res 2009; 19: 1141–1152.
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000; 30: 61–67.
Marsh TL . Terminal restriction fragment length polymorphism (T-RFLP): an emerging method for characterizing diversity among homologous populations of amplification products. Curr Opin Microbiol 1999; 2: 323–327.
Muyzer G . DGGE/TGGE a method for identifying genes from natural ecosystems. Curr Opin Microbiol 1999; 2: 317–322.
Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A . Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 2004; 97: 1166–1177.
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R . Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 2004; 70: 7220–7228.
Dethlefsen L, Huse S, Sogin ML, Relman DA . The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6: e280.
Vacharaksa A, Finlay BB . Gut microbiota: metagenomics to study complex ecology. Curr Biol 2010; 20: R569–R571.
Preidis GA, Versalovic J . Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 2009; 136: 2015–2031.
Turnbaugh PJ, Gordon JI . An invitation to the marriage of metagenomics and metabolomics. Cell 2008; 134: 708–713.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Torrazza, R., Neu, J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 31 (Suppl 1), S29–S34 (2011). https://doi.org/10.1038/jp.2010.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2010.172