Abstract
Placental growth factor (PlGF) is an increasingly important molecule in the prediction, diagnosis and treatment of pre-eclampsia. It has pro-angiogenic effects on the feto-placental circulation and supports trophoblast growth. Mechanisms by which PlGF expression is regulated continue to be investigated. Low circulating PlGF precedes the manifestation of clinical disease in pre-eclamptic pregnancies and intrauterine growth restriction. This suggests that low PlGF is a marker of abnormal placentation, but it remains uncertain whether this is a cause or consequence. Prediction of pre-eclampsia using PlGF is promising and may assist in the targeting of resources to women at highest risk of adverse pregnancy outcomes. Promisingly, experimental animal models of pre-eclampsia have been successfully treated with supplemental PlGF. Treatment of pre-eclampsia with PlGF is a potential therapeutic option requiring further exploration. This review focuses specifically on the role of PlGF in normal and pathological placental development and in the clinical management of pre-eclampsia.
Similar content being viewed by others
Introduction
Placental growth factor (PlGF) is an increasingly important player in the clinical management of pre-eclampsia. This review outlines the role of PlGF in human physiology but focuses specifically on the current understanding of PlGF’s function in normal and pathological placental development. The differences in peripheral concentrations of PlGF between normal and pre-eclamptic pregnancy are highlighted and utility of PlGF as a predictive or diagnostic test for pre-eclampsia is discussed. Finally, the possibility of PlGF as treatment for pre-eclampsia is considered.
PlGF
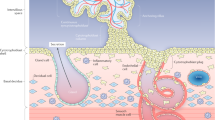
Placental growth factor is a member of the vascular endothelial growth factor (VEGF) family and is predominantly expressed in the placenta, although it is also expressed at low levels in many other tissues, including the heart, lung, thyroid, liver, skeletal muscle and bone. The human PlGF gene is located on chromosome 14q14 and encodes 4 isoforms of PlGF. The protein is secreted as a glycosylated homodimer and PlGF-1 and -3 are diffusible isoforms whereas PlGF-2 and PlGF-4 have heparin binding domains. Of these, PlGF-1 and -2 are the most abundant forms, and during pregnancy they are secreted in a strongly correlated manner, indicating a common regulation mechanism. The presence of a heparin binding domain suggests that PlGF-2 and -4 remain cell membrane-associated and act in an autocrine fashion, while the diffusible forms of PlGF probably affect targets in a paracrine manner. Mice produce only the PlGF-2 variant. Placental growth factor binds to VEGFR-1 (vascular endothelial growth factor-1 receptor-1) or FLT-1 (fms-related tyrosine kinase-1) and its soluble variant sFLT-1 (soluble fms-like tyrosine kinase-1), but not VEGFR-2 (vascular endothelial growth factor receptor-2), also known as KDR (kinase insert domain receptor) or FLK-1 (foetal liver kinase-1) (Figure 1). It also binds to neuropilin receptor-1 (NP-1) and -2 present in neurons. NP-1 has also recently been identified in placenta but its role is yet to be elucidated.1
PlGF and angiogenesis
Angiogenesis is a vital process for embryonic development and growth is regulated by a complex interplay of a multitude of factors, including the VEGF family. New blood vessels form by remodelling of existing vasculature with sprouting of new branches followed by non-branching angiogenesis—elongation and enlargement. In human adult life, angiogenesis occurs predominantly in the endometrium during the menstrual cycle, in wound healing and as an adaptive process in the myocardium and skeletal muscle. Placental growth factor is pro-angiogenic as it enhances the activity of VEGF by competitively binding to the VEGFR-1 receptor, allowing VEGF to bind then to VEGFR-2 which has stronger tyrosine kinase activity. However, PlGF also exerts it’s affect through other mechanisms such as intermolecular transphosphorylation of VEGFR-2 following activation of VEGFR-1, which amplifies VEGFR-2 response to VEGF binding. Additionally, PlGF forms a heterodimer with VEGF, which may have either pro- or anti-angiogenic effects.
The main role of PlGF in tissues other than the placenta is angiogenesis in response to pathological ischaemia or injury. Knockout mice (PlGF−/−) have impaired angiogenesis and arteriogenesis during pathological conditions such as heart, limb and ocular ischaemia. Physiological exercise induced ischaemia in skeletal and cardiac muscle does not stimulate PlGF production but PlGF is upregulated in pathological conditions such as coronary artery disease. Accordingly, PlGF expression is upregulated by hypoxia in non-trophoblast cells.2 In contrast, transcriptional activity of PlGF in trophoblast is suppressed by hypoxia3 and increased by a normoxic environment pointing to a specific regulatory mechanism and function in these cells.2
In tumour cells, PlGF expression is part of the angiogenic switch that supports tumour vascularisation. In most cancers (solid and haematological), there is a positive correlation between cancer severity and PlGF expression and protein levels, with an inverse relationship between PlGF and survival. The role of PlGF is context dependent, varying according to tumour type and stage and consequently tumour reduction with PlGF inhibition has not been demonstrated consistently. For example, overexpression of PlGF can decrease rather than increase angiogenesis as formation of VEGF/PLGF heterodimers are less pro-angiogenic than VEGF homodimers4 and, in another study, blockade of PlGF seemed to improve the tumour vasculature quality.5 The specific expression of PlGF in diseased tissues presents itself as a good opportunity for targeted therapy. A phase 1 study of an anti-PlGF monoclonal antibody in combination with anti-VEGF antibody bevacizumab in patients with recurrent glioblastoma multiforme did not show improved survival.6 Two further studies have been conducted in patients with hepatocellular carcinoma and metastatic colorectal or ovarian cancer but results have not been reported.
PlGF in reproduction
The role of PlGF in reproduction is still being understood. PlGF is thought to be redundant in reproduction as PlGF knockout mice are fertile and pups have similar growth potential compared to wild type mice. However, endometrial tissue during the secretory phase of the human menstrual cycle has been shown to secrete PlGF.7 The presence of PlGF during this window supports a role of PlGF in influencing embryo implantation, but this has yet to be further characterised. Although PLGF knockout mice appear normal, differences in foetal and adult brain development8 have recently been demonstrated. The preliminary data in the children of pre-eclamptic women suggest subtle differences in brain vascular development, which is thought to be related to intrauterine events.9 Therefore, while PlGF may not be essential to reproduction it is still likely to be an important influence on pregnancy and vascular development.
The role of PlGF in placental development
Circulating PlGF is prominently elevated in pregnancy with the source being the placenta. The function of PlGF in the placenta is likely to be in the promotion of development and maturation of the placental vascular system.
Implantation sites of PlGF knockout mice show abnormal placental vasculature.10 There is decreased branching in the anti-mesometrial (feto-placental) vessels and increased lacunarity, indicating a lack of uniformity of vessel formation. Utero-placental vessels also display decreased branching, but decidual invasion is not influenced. Mouse uterine lymphatic vessels are also abnormally developed. In human placenta, expression of PlGF corresponds with different stages in placental development with non-branching angiogenesis of the feto-placental circulation and maturation of the utero-placental circulation coinciding with increased expression of PlGF in later gestation. Evidently, development of the placental circulation is influenced by PlGF even though the absence of this angiogenic factor does not result in death in offspring of knockout mice. In contrast, a mouse knock-in with PlGF expressed in T cells showed decreased angiogenesis in offspring,11 suggesting that, analogous to the variable role of PlGF in a tumour life cycle, the effect of PlGF in pregnancy is situation specific.
When postulating possible effects in human pregnancy from the findings in rodent studies, differences in placental physiology between species must be noted. Although abnormal implantation evident in PlGF knockout mice may not lead to embryonic or foetal death, the consequences of aberrant placental development may not be readily apparent in mice. Unlike human pregnancy, mouse placenta does not invade into the myometrium and endovascular invasion is limited. Consequently, abnormal spiral artery remodelling in mice does not lead to placental insufficiency or abnormal blood pressure regulation.12 Processes in human placenta development may be more extensively mediated by PlGF.
Placental expression of PlGF dominates from the second trimester when the utero-placental circulation is advancing, with myometrial spiral arteries remodelling in a ‘second wave’ of invasion beginning at 16–18 weeks’ gestation. However, there have been conflicting reports as to whether PlGF contributes to trophoblast invasion.13, 14 Trophoblast develop invasive characteristics in response to increased oxygen tension and PlGF expression also increases with improved placental oxygenation, but it is uncertain whether these two events have a directly linked regulatory mechanism. Differentiation of uterine natural killer cells is influenced by PlGF15 and these cells may in turn mediate trophoblast invasion into the decidua.
PlGF increases proliferation of trophoblast cells. It also reduces apoptosis of trophoblast cells16 when these cells are starved, but not when they are exposed to inflammatory cytokines.17 This may manifest as increased circulating trophoblast debris found in cases of pre-eclampsia (where there is often PlGF deficiency), but the exact role of PlGF mediated reduction of apoptosis in placental development is not clear.
PlGF levels in normal pregnancy
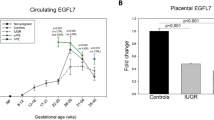
Concentrations of PlGF are low in the first trimester of an uncomplicated pregnancy and increases from week 11 to 12 onwards to a peak at week 30, after which it decreases (Figure 2). This is in contrast with sFLT-1, which increases towards the completion of pregnancy. This normal divergence of angiogenic factors levels occurs as the bioavailability of PlGF is reduced by binding to sFLT-1. Normal PlGF concentrations are dependent on gestational age, with the lower limit of normal (defined as the 5th centile) ranging from a peak of approximately 141 pg ml−1 at around 30 weeks gestation to 23 pg ml−1 at term.18
Circulating PlGF concentrations gradually increase during pregnancy to reach a peak at ~30 weeks gestation. In pre-eclampsia PlGF concentrations are comparatively lower throughout pregnancy. Placental expression of PlGF dominates from the second trimester of pregnancy, coinciding with non-branching angiogenesis of feto-placental vessels and maturation of the utero-placental circulation. Placental growth factor may contribute to trophoblast invasion, increase trophoblast proliferation and reduce apoptosis.
PlGF in pre-eclampsia
Serum and urinary PlGF is found to be decreased in women both at the time of diagnosis with pre-eclampsia and well in advance of syndrome onset. The deficiency in PlGF is likely due to a combination of decreased expression of PlGF and reduced free PlGF due to binding with sFLT-1, which is elevated in affected women.19 In early pregnancy, PlGF concentrations are lower in women who subsequently develop preclampsia than in normal pregnant women, but sFLT-1 levels are no different, suggesting that PlGF expression in the placenta is decreased. However, towards completion of pregnancy, there is a reciprocal relationship between sFLT-1 and PlGF with rising levels of total (free and bound to VEGF or PlGF) sFLT-1 and lower free PlGF levels.20 This suggests that in the latter half of pregnancy, low PlGF concentrations occur predominantly due to sequestering of PlGF by sFLT-1.
Low circulating PlGF is probably both a consequence of abnormal early events in placentation and a contributing factor to continued abnormal growth during the latter half of pregnancy. The hypothesis that PlGF is an indicator of abnormal placentation is supported by the observation that women without pre-eclampsia who give birth to small for gestational age babies also have low PlGF early in pregnancy.21 The data regarding the expression of PlGF in placental tissue is conflicting. Expression of PlGF is postulated to be lowered due to suppression by persistent placental hypoxia resulting from an underdeveloped utero-placental circulation. However, studies have also shown increased3 or no change in PlGF expression22 in pre-eclamptic placental tissue. Regulation of PlGF expression is unclear, but several mechanisms have been explored such as endoplasmic reticulum stress and epigenetic changes altering the effect of the transcription factor hypoxia-inducible factor-1α (HIF1-α), although the role of HIF1-α in trophoblast growth is debated.2, 23, 24 Inflammation may also influence PlGF expression as PlGF concentrations are elevated in sepsis.
PlGF for the prediction and diagnosis of pre-eclampsia
Despite intense research efforts, the diagnosis and management pre-eclampsia has remained unchanged for decades. Recognition of the differences in circulating angiogenic factor levels between pre-eclamptic and normal pregnancies has resulted in investigation into whether these factors can identify women who require close monitoring. Once the diagnosis of pre-eclampsia is made, only delivery of the placenta can alleviate the condition.
In women who will develop pre-eclampsia, PlGF is low in the first trimester, well before the disease clinically manifests. Despite the differences between groups, single angiogenic factors are not useful for prediction25 with PlGF having a sensitivity of 32% for a 5% false-positive rate. Combinations of angiogenic factors such as the sFLT-1:PlGF ratio with aspects of history or ultrasound findings to create multifactorial predictive tools are promising but not currently in wide usage. For example the Fetal Medicine Foundation predictive algorithm at 11–13 weeks gestation,26 which uses a combination of maternal characteristics, mean arterial pressure, uterine artery pulsatility index, PAPP-A and PlGF detects 95 and 46% of women with early and late pre-eclampsia, respectively with a false-positive rate of 10%.27 Application of prediction algorithms in specific subgroups such as women with antiphospholipid syndrome and systemic lupus erythematosus may be more successful due to the increased baseline risk of adverse pregnancy outcome and potentially greater importance of angiogenic factors in the pathogenesis of disease in these patients.28
The utility of PlGF and other angiogenic factors VEGF, and sFLT-1 in prediction pre-eclampsia is likely limited by the heterogeneity of pathology that underlies the spectrum of clinical presentation of pre-eclampsia. Affected women range from those with early-onset disease and severe intrauterine growth restriction to others with mild symptoms presenting at term. Early, severe disease appears to be more strongly associated with abnormal placentation and abnormalities in angiogenic factors are more pronounced in these patients. Persistently low levels of PlGF throughout pregnancy and abnormal sFLT-1: PlGF ratio identifies a subset of women with an early and more severe presentation of the disease. The use of angiogenic factors may be in categorising pre-eclamptic patients to allow more directed research specific to subtypes of pre-eclampsia.
In women suspected of having pre-eclampsia, but not yet meeting diagnostic criteria, the sFLT-1: PlGF ratio or plasma PlGF alone is useful as a ‘rule out’ test with a high negative predictive value. Maternal plasma PlGF less than the 5th centile for gestation at the time of presentation performed better than a 5 factor combination of commonly used clinical parameters (systolic and diastolic blood pressure, alanine transferase, uric acid and dipstick proteinuria) (ROC area 0.87 vs 0.70 P<0.001) in diagnosing women with pre-eclampsia requiring delivery within 2 weeks.29 The sensitivity of low PlGF was highest for the delivery of a small for gestational age infant, further supporting the observation that low PlGF reflects placental disease.
Cost-benefit analyses of these tests suggest that angiogenic factor testing will be useful in determining appropriate resource allocation by allowing reduced frequency of observation of women deemed at low risk of developing pre-eclampsia. The sFLT-1: PlGF ratio is also useful for distinguishing patient with pre-eclampsia from those with conditions that may present similarly such as glomerulonephritis.
Treatment of experimental models of pre-eclampsia with PlGF
The clinical presentation of pre-eclampsia is a consequence of widespread endothelial dysfunction triggered in part by excess sFLT-1. Binding of local VEGF by sFLT-1 in tissues with high expression of VEGF such as the kidney and liver appears to be responsible for the clinical signs such as proteinuria and raised transaminases. While decreased circulating PlGF does not appear to directly contribute to the clinical syndrome, supplementation of PlGF to correct the angiogenic balance and act as a ligand for excess sFLT-1 has been considered as a possible treatment avenue. Similarly, restoration of the angiogenic factor imbalance by removal of sFLT-1 by apheresis has been attempted with promising results.30
There are currently four reports of PlGF treatment in experimental models of pre-eclampsia. Two different rodent models have been successfully treated with exogenous PlGF. Suzuki et al.31 established experimental pre-eclampsia by transfection of mice with an adenovirus to increase sFLT-1. Mouse PlGF-2 was given intraperitoneally for two days, which resulted in the reduction of hypertension but not proteinuria. Spradley et al.32 and Zhu et al33 used a rat reduced uterine placental perfusion (RUPP) model, which is shown to result in an increase sFLT-1. Spradley et al. administered recombinant human PlGF (rhPlGF) by continuous infusion via an intraperitoneal osmotic pump. This reduced blood pressure, proteinuria and improved glomerular filtration rate in addition to reducing markers of oxidative stress. sFLT-1 levels also decreased significantly. Zhu et al. infused rhPlGF both intravenously and via subcutaneously resulting in blood pressure reduction. Similarly, in a non-human primate utero-placental ischemic model of pre-eclampsia, the administration of rhPLGF reduced blood pressure and proteinuria.34 The concentration of PlGF decreases in non-human primates with utero-placental ischaemia but sFLT-1 levels remained elevated in animals that were administered PlGF despite improvement of clinical signs. The conflicting changes in sFLT-1 levels in the RUPP model in rodents as compared with the UPI model in non-human primates may result from differences in ELISA specificity. Commercial kits may measure free sFLT-1 or sFLT-1 bound to VEGF or PlGF. The mouse sFLT-1 kit used by Spradley et al. measures free sFLT-1, suggesting that rhPlGF binds with sFLT-1 and reduces free circulating sFLT-1.32 A direct effect of PlGF upon placental or extra-placental release of sFLT-1 remains a possibility.
Pravastatin has been demonstrated to increase PlGF in a lentivirus human sFLT-1 transfected mouse model.35 There was reduction in blood pressure and placental and foetal weights normalised.35 This may be because of the increase in PlGF; however, pravastatin also has been shown to dampen inflammatory cytokines TNF-α and IL-1 and reduce vascular reactivity which may also account for this observation. At least two clinical trials are currently underway to determine the utility of pravastatin in pre-eclampsia prevention: ‘Statins to ameliorate early-onset preeclampsia’ (www.controlled-trials.com ISRCTN23410175) and ‘Pravastatin for prevention of preeclampsia’ (www.ClinicalTrials.gov NCT01717586).
Future directions
Treatment of pre-eclampsia with PlGF is promising but many uncertainties remain. The delicate balance of anti- and pro-angiogenic factors, presumably pathological in the case of pre-eclampsia, may still be significantly disrupted by exogenous PlGF. Although low PlGF concentrations have been identified in women who subsequently develop pre-eclampsia, the normal range of PlGF is wide in healthy pregnant women making interpretation individual cases difficult. The concentration to which PlGF should be restored for individual patients is also uncertain. Timing and duration of exposure to exogenous PlGF for treatment is also important given the usual decline in PlGF concentrations during normal pregnancy.
Off target effects of PlGF are of concern as the VEGFR-1 receptor is present in many tissues. However, as PlGF does not bind to VEGFR-2, it is likely that any unintended effects will not be as significant as compared with VEGF, which causes hypotension and increased vascular permeability mediated by VEGFR-2. Although high circulating PlGF present during pregnancy (as compared to in the non-pregnant state) does not appear to cause abnormalities in other tissues, peripheral administration of PlGF may have untoward effects such as promotion of inflammation and malignancy. A concern would be the effect of this molecule upon the foetus as transgenic mice with PlGF overexpression in T cells displayed impaired angiogenesis, resulting in death during gestation and growth restriction even after birth.11
Despite these limitations, restoration of angiogenic factor balance could be a means of treating pre-eclampsia. It has the potential to prolong pregnancy in a condition for which there is currently no definitive treatment excepting delivery of the placenta, even if this means prematurity. Reversal of endothelial dysfunction induced by excess sFLT-1 may be partially achieved by closely titrated replacement of PlGF or removal of sFLT-1 by apheresis. This is a biologically plausible avenue for therapeutic development and needs to be further explored.
References
Arad A, Nammouz S, Nov Y, Ohel G, Bejar J, Vadasz Z . The Expression of neuropilin-1 in human placentas from normal and preeclamptic pregnancies. Int J Gynecol Pathol 2016.
Gobble RM, Groesch KA, Chang M, Torry RJ, Torry DS . Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta 2009; 30 (10): 869–875.
Khaliq A, Dunk C, Jiang J, Shams M, Li XF, Acevedo C et al. Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for ‘placental hyperoxia’ in intrauterine growth restriction. Lab Invest 1999; 79 (2): 151–170.
Xu L, Cochran DM, Tong RT, Winkler F, Kashiwagi S, Jain RK et al. Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res 2006; 66 (8): 3971–3977.
Van de Veire S, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, Schmidt T et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 2010; 141 (1): 178–190.
Lassen U, Chinot OL, McBain C, Mau-Sorensen M, Larsen VA, Barrie M et al. Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro Oncol 2015; 17 (7): 1007–1015.
Binder NK, Evans J, Salamonsen LA, Gardner DK, Kaitu'u-Lino TJ, Hannan NJ . Placental growth factor is secreted by the human endometrium and has potential important functions during embryo development and implantation. PLoS ONE 2016; 11 (10): e0163096.
Luna RL, Kay VR, Ratsep MT, Khalaj K, Bidarimath M, Peterson N et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol Hum Reprod 2016; 22 (2): 130–142.
Dang F, Croy BA, Stroman PW, Figueiro-Filho EA . Impacts of Preeclampsia on the Brain of the Offspring. Rev Bras Ginecol Obstet 2016; 38 (8): 416–422.
Ratsep MT, Carmeliet P, Adams MA, Croy BA . Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta 2014; 35 (9): 772–775.
Kang MC, Park SJ, Kim HJ, Lee J, Yu DH, Bae KB et al. Gestational loss and growth restriction by angiogenic defects in placental growth factor transgenic mice. Arterioscler Thromb Vasc Biol 2014; 34 (10): 2276–2282.
Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension 2010; 55 (3): 729–737.
Athanassiades A, Lala PK . Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 1998; 19 (7): 465–473.
Knuth A, Liu L, Nielsen H, Merril D, Torry DS, Arroyo JA . Placenta growth factor induces invasion and activates p70 during rapamycin treatment in trophoblast cells. Am J Reprod Immunol 2014; 73: 330–340.
Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P et al. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol 2007; 178 (7): 4267–4275.
Arroyo J, Price M, Straszewski-Chavez S, Torry RJ, Mor G, Torry DS . XIAP protein is induced by placenta growth factor (PLGF) and decreased during preeclampsia in trophoblast cells. Syst Biol Reprod Med 2014; 60 (5): 263–273.
Desai J, Holt-Shore V, Torry RJ, Caudle MR, Torry DS . Signal transduction and biological function of placenta growth factor in primary human trophoblast. Biol Reprod 1999; 60 (4): 887–892.
Saffer C, Olson G, Boggess KA, Beyerlein R, Eubank C, Sibai BM . Determination of placental growth factor (PlGF) levels in healthy pregnant women without signs or symptoms of preeclampsia. Pregnancy Hypertens 2013; 3 (2): 124–132.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350 (7): 672–683.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111 (5): 649–658.
Poon LC, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH . Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenat Diagn 2008; 28 (12): 1110–1115.
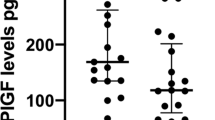
Hoeller A, Ehrlich L, Golic M, Herse F, Perschel FH, Siwetz M et al. Placental expression of sFlt-1 and PlGF in early preeclampsia vs. early IUGR vs. age-matched healthy pregnancies. Hypertens Pregnancy 2017; 36 (2): 151–160.
Mizuuchi M, Cindrova-Davies T, Olovsson M, Charnock-Jones DS, Burton GJ, Yung HW . Placental endoplasmic reticulum stress negatively regulates transcription of placental growth factor via ATF4 and ATF6beta: implications for the pathophysiology of human pregnancy complications. J Pathol 2016; 238 (4): 550–561.
Tudisco L, Della Ragione F, Tarallo V, Apicella I, D'Esposito M, Matarazzo MR et al. Epigenetic control of hypoxia inducible factor-1alpha-dependent expression of placental growth factor in hypoxic conditions. Epigenetics 2014; 9 (4): 600–610.
Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. Bjog 2012; 119 (7): 778–787.
Poon LC, Syngelaki A, Akolekar R, Lai J, Nicolaides KH . Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther 2013; 33 (1): 16–27.
Park FJ, Leung CH, Poon LC, Williams PF, Rothwell SJ, Hyett JA . Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust N Z J Obstet Gynaecol 2013; 53 (6): 532–539.
Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol 2016; 214 (1): 108 e1–108 e14.
Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013; 128 (19): 2121–2131.
Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA et al. Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 2016; 27 (3): 903–913.
Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M et al. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension 2009; 54 (5): 1129–1135.
Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA et al. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 2016; 67 (4): 740–747.
Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA . Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol 2016; 311 (3): R505–R521.
Makris A, Yeung KR, Lim SM, Sunderland N, Heffernan S, Thompson JF et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension 2016; 73 (4): 330–340.
Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA 2011; 108 (4): 1451–1455.
Acknowledgements
KC is supported by a National Health Medical Research Council Postgraduate Scholarship GNT1075337.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Chau, K., Hennessy, A. & Makris, A. Placental growth factor and pre-eclampsia. J Hum Hypertens 31, 782–786 (2017). https://doi.org/10.1038/jhh.2017.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2017.61
This article is cited by
-
RNA-seq reveals differentially expressed lncRNAs and circRNAs and their associated functional network in HTR-8/Svneo cells under hypoxic conditions
BMC Medical Genomics (2024)
-
Excessive endometrial PlGF- Rac1 signalling underlies endometrial cell stiffness linked to pre-eclampsia
Communications Biology (2024)
-
Angiogenic factors for early prediction of preeclampsia
Hypertension Research (2024)
-
Soluble FLT-1 in angiogenesis: pathophysiological roles and therapeutic implications
Angiogenesis (2024)
-
Long-Term Predictors of Gestational Hypertension: Placental Growth Factor, Pregnancy-Associated Plasma Protein-A and Free Beta-Hcg Versus Mean Arterial Pressure and Uterine Artery Doppler Versus a Combination of Both: A Comparative Study
The Journal of Obstetrics and Gynecology of India (2024)