Abstract
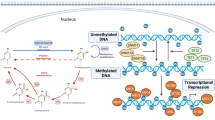
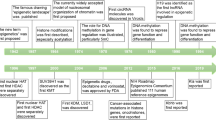
The major epigenetic features of mammalian cells include DNA methylation, posttranslational histone modifications and RNA-based mechanisms including those controlled by small non-coding RNAs (microRNAs (miRNAs)). An important aspect of epigenetic mechanisms is that they are potentially reversible and may be influenced by nutritional–environmental factors and through gene–environment interactions. Studies on epigenetic modulations could help us understand the mechanisms involved in essential hypertension and further prevent it’s progress. This review is focused on new knowledge on the role of epigenetics, from DNA methylation to miRNAs, in essential hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pausova Z, Tremblay J, Hamet P . Gene-environment interactions in hypertension. Curr Hypertens Rep 1999; 1 (1): 42–50.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34 (28): 2159–2219.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365 (9455): 217–223.
Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002; 288 (15): 1882–1888.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J et al. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014 311 (5): 507–520.
Oparil S, Zaman MA, Calhoun DA . Pathogenesis of hypertension. Ann Intern Med 2003; 139 (9): 761–776.
Ehret GB . Genome-wide association studies: contribution of genomics to understanding blood pressure and essential hypertension. Curr Hypertens Rep 2010; 12 (1): 17–25.
Kunes J, Zicha J . The interaction of genetic and environmental factors in the etiology of hypertension. Physiol Res 2009; 58 (Suppl 2): S33–S41.
Millis RM . Epigenetics and hypertension. Curr Hypertens Rep 2011; 13 (1): 21–28.
Udali S, Guarini P, Moruzzi S, Choi SW, Friso S . Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med 2013; 34 (4): 883–901.
Webster AL, Yan MS, Marsden PA . Epigenetics and cardiovascular disease. Can J Cardiol 2013; 29 (1): 46–57.
Ordovás JM, Smith CE . Epigenetics and cardiovascular disease. Nat Rev Cardiol 2010; 7 (9): 510–519.
Miranda TB, Jones PA . DNA methylation: the nuts and bolts of repression. J Cell Physiol 2007; 213 (2): 384–390.
Rottach A, Leonhardt H, Spada F . DNA methylation-mediated epigenetic control. J Cell Biochem 2009; 108 (1): 43–51.
Yan J, Zierath JR, Barrès R . Evidence for non-CpG methylation in mammals. Exp Cell Res 2011; 317 (18): 2555–2561.
Mariniello B, Ronconi V, Sardu C, Pagliericcio A, Galletti F, Strazzullo P et al. Analysis of the 11beta-hydroxysteroid dehydrogenase type 2 gene (HSD11B2) in human essential hypertension. Am J Hypertens 2005; 18 (8): 1091–1098.
Frey FJ, Odermatt A, Frey BM . Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens 2004; 13 (4): 451–458.
Ferrari P . The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim Biophys Acta 2010; 1802 (12): 1178–1187.
Campino C, Carvajal CA, Cornejo J, San Martín B, Olivieri O, Guidi G et al. 11β-Hydroxysteroid dehydrogenase type-2 and type-1 (11β-HSD2 and 11β-HSD1) and 5β-reductase activities in the pathogenia of essential hypertension. Endocrine 2010; 37 (1): 106–114.
Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM . Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest 2004; 114 (8): 1146–1157.
Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V et al. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis 2008; 199 (2): 323–327.
Howard TE, Shai SY, Langford KG, Martin BM, Bernstein KE . Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol 1990; 10 (8): 4294–4302.
Andrade MC, Quinto BM, Carmona AK, Ribas OS, Boim MA, Schor N et al. Purification and characterization of angiotensin I-converting enzymes from mesangial cells in culture. J Hypertens 1998; 16 (12 Pt 2): 2063–2074.
Duncan AM, Burrell LM, Kladis A, Campbell DJ . Effects of angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptides in rats with myocardial infarction. J Cardiovasc Pharmacol 1996; 28 (6): 746–754.
Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P et al. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension 2004; 43 (4): 854–859.
Riviere G, Lienhard D, Andrieu T, Vieau D, Frey BM, Frey FJ . Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 2011; 6 (4): 478–489.
Rangel M, dos Santos JC, Ortiz PH, Hirata M, Jasiulionis MG, Araujo RC et al. Modification of epigenetic patterns in low birth weight children: importance of hypomethylation of the ACE gene promoter. PLoS ONE 2014; 9 (8): e106138.
Gamba G . Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 2005; 85 (2): 423–493.
Chipperfield AR, Harper AA . Chloride in smooth muscle. Prog Biophys Mol Biol 2000; 74 (3-5): 175–221.
Orlov SN, Tremblay J, Hamet P . NKCC1 and hypertension: a novel therapeutic target involved in the regulation of vascular tone and renal function. Curr Opin Nephrol Hypertens 2010; 19 (2): 163–168.
Anfinogenova YJ, Baskakov MB, Kovalev IV, Kilin AA, Dulin NO, Orlov SN . Cell-volume-dependent vascular smooth muscle contraction: role of Na+, K+, 2Cl− cotransport, intracellular Cl- and L-type Ca2+ channels. Pflugers Arch 2004; 449 (1): 42–55.
Giménez I . Molecular mechanisms and regulation of furosemide-sensitive Na-K-Cl cotransporters. Curr Opin Nephrol Hypertens 2006; 15 (5): 517–523.
Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 2005; 280 (52): 42685–42693.
Zagórska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 2007; 176 (1): 89–100.
Richardson C, Alessi DR . The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 2008; 121 (Pt 20): 3293–3304.
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 2006; 397 (1): 223–231.
Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P et al. WNK1 regulates vasoconstriction and blood pressure response to α 1-adrenergic stimulation in mice. Hypertension 2011; 58 (3): 439–445.
Lee HA, Baek I, Seok YM, Yang E, Cho HM, Lee DY et al. Promoter hypomethylation upregulates Na+-K+-2Cl− cotransporter 1 in spontaneously hypertensive rats. Biochem Biophys Res Commun 2010; 396 (2): 252–257.
Cho HM, Lee HA, Kim HY, Han HS, Kim IK . Expression of Na+-K+ −2Cl− cotransporter 1 is epigenetically regulated during postnatal development of hypertension. Am J Hypertens 2011; 24 (12): 1286–1293.
Casari G, Barlassina C, Cusi D, Zagato L, Muirhead R, Righetti M et al. Association of the alpha-adducin locus with essential hypertension. Hypertension 1995; 25 (3): 320–326.
Matsuoka Y, Li X, Bennett V . Adducin: structure, function and regulation. Cell Mol Life Sci 2000; 57 (6): 884–895.
Manunta P, Barlassina C, Bianchi G . Adducin in essential hypertension. FEBS Lett 1998; 430 (1–2): 41–44.
Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L et al. Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest 1996; 97 (12): 2815–2822.
Zhang LN, Liu PP, Wang L, Yuan F, Xu L, Xin Y et al. Lower ADD1 gene promoter DNA methylation increases the risk of essential hypertension. PLoS ONE 2013; 8 (5): e63455.
Zhang YC, Bui JD, Shen L, Phillips MI . Antisense inhibition of beta(1)-adrenergic receptor mRNA in a single dose produces a profound and prolonged reduction in high blood pressure in spontaneously hypertensive rats. Circulation 2000; 101 (6): 682–688.
Kong H, Li X, Zhang S, Guo S, Niu W . The β1-adrenoreceptor gene Arg389Gly and Ser49Gly polymorphisms and hypertension: a meta-analysis. Mol Biol Rep 2013; 40 (6): 4047–4053.
Wang H, Liu J, Liu K, Liu Y, Wang Z, Lou Y et al. β1-adrenoceptor gene Arg389Gly polymorphism and essential hypertension risk in general population: a meta-analysis. Mol Biol Rep 2013; 40 (6): 4055–4063.
Jiang Q, Yuan H, Xing X, Liu J, Huang Z, Du X . Methylation of adrenergic β1 receptor is a potential epigenetic mechanism controlling antihypertensive response to metoprolol. Indian J Biochem Biophys 2011; 48 (5): 301–307.
Zhang D, Yu ZY, Cruz P, Kong Q, Li S, Kone BC . Epigenetics and the control of epithelial sodium channel expression in collecting duct. Kidney Int 2009; 75 (3): 260–267.
Bhalla V, Hallows KR . Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 2008; 19 (10): 1845–1854.
Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D et al. Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 2010; 21 (11): 1942–1951.
Thomas CP, Itani OA . New insights into epithelial sodium channel function in the kidney: site of action, regulation by ubiquitin ligases, serum- and glucocorticoid-inducible kinase and proteolysis. Curr Opin Nephrol Hypertens 2004; 13 (5): 541–548.
Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D et al. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 2007; 117 (3): 773–783.
Stokes JB, Sigmund RD . Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol 1998; 274 (6 Pt 1): C1699–C1707.
Yu Z, Kong Q, Kone BC . Aldosterone reprograms promoter methylation to regulate αENaC transcription in the collecting duct. Am J Physiol Renal Physiol 2013; 305 (7): F1006–F1013.
Margueron R, Reinberg D . Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 2010; 11 (4): 285–296.
Goldberg AD, Allis CD, Bernstein E . Epigenetics: a landscape takes shape. Cell 2007; 128 (4): 635–638.
Kang TJ, Yuzawa S, Suga H . Expression of histone H3 tails with combinatorial lysine modifications under the reprogrammed genetic code for the investigation on epigenetic markers. Chem Biol 2008; 15 (11): 1166–1174.
Woldańska-Okońska M, Czernicki J . [Biological effects produced by the influence of low frequency electromagnetic fields on hormone secretion]. Przegl Lek 2003; 60 (10): 657–662.
Irmak MK, Sizlan A . Essential hypertension seems to result from melatonin-induced epigenetic modifications in area postrema. Med Hypotheses 2006; 66 (5): 1000–1007.
Sharma R, Ottenhof T, Rzeczkowska PA, Niles LP . Epigenetic targets for melatonin: induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J Pineal Res 2008; 45 (3): 277–284.
Morimoto S, Sasaki S, Itoh H, Nakata T, Takeda K, Nakagawa M et al. Sympathetic activation and contribution of genetic factors in hypertension with neurovascular compression of the rostral ventrolateral medulla. J Hypertens 1999; 17 (11): 1577–1582.
Mao J, Li C, Zhang Y, Li Y, Zhao Y . Human with-no-lysine kinase-4 3′-UTR acting as the enhancer and being targeted by miR-296. Int J Biochem Cell Biol 2010; 42 (9): 1536–1543.
Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 2003; 35 (4): 372–376.
Nguyen Dinh Cat A, Ouvrard-Pascaud A, Tronche F, Clemessy M, Gonzalez-Nunez D, Farman N et al. Conditional transgenic mice for studying the role of the glucocorticoid receptor in the renal collecting duct. Endocrinology 2009; 150 (5): 2202–2210.
Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F et al. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 2011; 17 (5): 573–580.
Li M, Zhao Y, Li Y, Li C, Chen F, Mao J et al. Upregulation of human with-no-lysine kinase-4 gene expression by GATA-1 acetylation. Int J Biochem Cell Biol 2009; 41 (4): 872–878.
Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC . Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem 2006; 281 (26): 18059–18068.
Lee JY, Prineas RJ, Eaton JW . Heritability of erythrocyte sodium permeability: a possible genetic marker for hypertension. Ann Clin Lab Sci 2009; 39 (3): 241–250.
Reisenauer MR, Anderson M, Huang L, Zhang Z, Zhou Q, Kone BC et al. AF17 competes with AF9 for binding to Dot1a to up-regulate transcription of epithelial Na+ channel alpha. J Biol Chem 2009; 284 (51): 35659–35669.
Lewis BP, Burge CB, Bartel DP . Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120 (1): 15–20.
Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A . Identification of mammalian microRNA host genes and transcription units. Genome Res 2004; 14 (10A): 1902–1910.
Jinek M, Doudna JA . A three-dimensional view of the molecular machinery of RNA interference. Nature 2009; 457 (7228): 405–412.
Liang M, Liu Y, Mladinov D, Cowley AW, Trivedi H, Fang Y et al. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 2009; 297 (3): F553–F558.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116 (2): 281–297.
Shukla GC, Singh J, Barik S . MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 2011; 3 (3): 83–92.
Clifford RL, Singer CA, John AE . Epigenetics and miRNA emerge as key regulators of smooth muscle cell phenotype and function. Pulm Pharmacol Ther 2013; 26 (1): 75–85.
Forman JJ, Legesse-Miller A, Coller HA . A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA 2008; 105 (39): 14879–14884.
Esteller M . Non-coding RNAs in human disease. Nat Rev Genet 2011; 12 (12): 861–874.
Mopidevi B, Ponnala M, Kumar A . Human angiotensinogen +11525 C/A polymorphism modulates its gene expression through microRNA binding. Physiol Genomics 2013; 45 (19): 901–906.
Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 2011; 58 (6): 1093–1098.
Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS . Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J Mol Cell Cardiol 2014; 75: 25–39.
Wang K, Long B, Zhou J, Li PF . miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem 2010; 285 (16): 11903–11912.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 2008; 15 (2): 272–284.
Bátkai S, Thum T . MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep 2012; 14 (1): 79–87.
Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE . MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J Am Soc Hypertens 2014; 8 (6): 368–375.
Santovito D, Mandolini C, Marcantonio P, De Nardis V, Bucci M, Paganelli C et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin Ther Targets 2013; 17 (3): 217–223.
Sayed AS, Xia K, Salma U, Yang T, Peng J . Diagnosis, prognosis and therapeutic role of circulating mirnas in cardiovascular diseases. Heart Lung Circ 2014; 23 (6): 503–510.
Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 2013; 14 (6): 11190–11207.
Acknowledgements
The study was supported by the Ministry of Science and Technology of China with ‘973’ grant 2012CB517804 and the National Natural Science Foundation of China with grants 81322002 and 81270333 to Dr Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, J., Gong, L., Tan, Y. et al. Hypertensive epigenetics: from DNA methylation to microRNAs. J Hum Hypertens 29, 575–582 (2015). https://doi.org/10.1038/jhh.2014.132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2014.132
This article is cited by
-
Association of APP gene polymorphisms and promoter methylation with essential hypertension in Guizhou: a case–control study
Human Genomics (2023)
-
Novel methylation mark and essential hypertension
Journal of Genetic Engineering and Biotechnology (2022)
-
Hypertensive disorders of pregnancy share common cfDNA methylation profiles
Scientific Reports (2022)
-
BKCa channel activity and vascular contractility alterations with hypertension and aging via β1 subunit promoter methylation in mesenteric arteries
Hypertension Research (2018)