Abstract
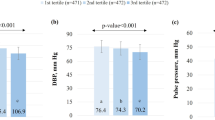
In the Dietary Approach to Stop Hypertension (DASH) trial, the DASH diet reduced blood pressure (BP) in a diverse sample of US adults. Subsequent analyses of this trial documented the efficacy of the DASH diet in several subgroups. Although subgroup analyses in individuals with metabolic syndrome (MS) have not been performed, the DASH diet has been recommended in MS patients. This paper is a subgroup analysis of the DASH trial, in which we examined the effect of study diets on BP in participants with and without MS. Participants were stratified according to MS status (99 with MS, 311 without MS (Non-MS)). The trial was a dietary intervention study in which participants were randomized to receive a control diet, a diet rich in fruits and vegetables, or the DASH diet. Outcomes were (i) the difference in BP between the end and the beginning of intervention and (ii) control of hypertension. We found no significant interaction between MS status and diet assignment on BP (each P-interaction >0.05). In the MS subgroup, the DASH diet compared with the control diet reduced systolic BP by 4.9 mm Hg (P=0.006) and diastolic BP by 1.9 mm Hg (P=0.15). In the Non-MS subgroup, corresponding net BP reductions were 5.2 mm Hg (P<0.001) and 2.9 mm Hg (P<0.001), respectively. The DASH diet controlled hypertension in 75% of hypertensive participants with MS (adjusted odds ratio=9.5 vs the control diet, P=0.05). In conclusion, the DASH diet similarly reduces BP in those with and without MS. Our findings provide direct evidence for existing recommendations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ford ES, Giles WH, Dietz WH . Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287 (3): 356–359.
Cameron A, Shaw J, Zimmet P . The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin N Am 2004; 33 (2): 351–375.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106 (25): 3143–3421.
Reaven GM . Banting Lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition 1997; 13 (1): 65 discussion 64, 66.
Mozumdar A, Liguori G . Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care 2011; 34 (1): 216–219.
Cheung BM, Wat NM, Man YB, Tam S, Cheng CH, Leung GM et al. Relationship between the metabolic syndrome and the development of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2). Am J Hypertens 2008; 21 (1): 17–22.
Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi D, Parodi A et al. Metabolic syndrome is associated with early signs of organ damage in nondiabetic, hypertensive patients. J Intern Med 2005; 257 (5): 454–460.
Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol 2004; 43 (10): 1817–1822.
Rossi R, Nuzzo A, Origliani G, Modena MG . Metabolic syndrome affects cardiovascular risk profile and response to treatment in hypertensive postmenopausal women. Hypertension 2008; 52 (5): 865–872.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336 (16): 1117–1124.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42 (6): 1206–1252.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112 (17): 2735–2752.
Whaley-Connell A, Palmer J, Sowers JR . Risk stratification and treatment options for patients with hypertension with metabolic syndrome and prediabetes. Adv Stud Med 2005; 5 (10C): S1011–S1018.
Ganne S, Arora SK, Dotsenko O, McFarlane SI, Whaley-Connell A . Hypertension in people with diabetes and the metabolic syndrome: pathophysiologic insights and therapeutic update. Curr Diab Rep 2007; 7 (3): 208–217.
Makaryus AN, Akhrass P, McFarlane SI . Treatment of hypertension in metabolic syndrome: implications of recent clinical trials. Curr Diab Rep 2009; 9 (3): 229–237.
Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995; 5 (2): 108–118.
Carroll MD, Abraham S, Dresser CM . Dietary Intake Source Data: United States, 1976–80. Vital and Health Statistics, Series 11, Data from the National Health Survey 1983, 1–483.
Ridker PM, Buring JE, Cook NR, Rifai N . C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107 (3): 391–397.
Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003; 108 (4): 414–419.
Alberti KG, Zimmet PZ . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15 (7): 539–553.
Dawson B, Trapp RG . Basic & Clinical Biostatistics 4th edn. Lange Medical Books-McGraw-Hill, Medical Pub. Division: New York, NY, USA, 2004.
Geller NL . Advances in Clinical Trial Biostatistics. Marcel Dekker: New York, NY, USA, 2004.
Lien LF, Brown AJ, Ard JD, Loria C, Erlinger TP, Feldstein AC et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension 2007; 50 (4): 609–616.
Karlsen A, Svendsen M, Seljeflot I, Laake P, Duttaroy AK, Drevon CA et al. Kiwifruit decreases blood pressure and whole-blood platelet aggregation in male smokers. J Hum Hypertens 2013; 27 (2): 126–130.
Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, Brader L et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome? a randomized study (SYSDIET). J Intern Med 2013.
Redon J, Cifkova R, Laurent S, Nilsson P, Narkiewicz K, Erdine S et al. The metabolic syndrome in hypertension: European society of hypertension position statement. J Hypertens 2008; 26 (10): 1891–1900.
Wong ND, Lopez VA, L’Italien G, Chen R, Kline SE, Franklin SS . Inadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003–2004. Arch Intern Med 2007; 167 (22): 2431–2436.
Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus—results of prospectively designed overviews of randomized trials. Arch Intern Med 2005; 165 (12): 1410–1419.
Wong ND, Pio JR, Franklin SS, L’Italien GJ, Kamath TV, Williams GR . Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol 2003; 91 (12): 1421–1426.
Acknowledgements
This work was supported by a Fulbright Grant (15043737) from the Institute of International Education to Dr Fadi Hikmat. This work was also supported by the late Mr Hikmat A Bihnam. The DASH clinical trial was supported by Grants (HL50981, HL50968, HL50972, HL50977, HL50982, HL02642, RR02635 and RR00722) from the National Heart, Lung and Blood Institute, the Office of Research on Minority Health and the National Center for Research Resources of the National Institutes of Health. We are indebted to the trial participants for their sustained commitment to DASH, and to the following companies, which donated food: Best Foods, Campbell’s Soup Company, Coca-Cola Foods Company, Comstock Michigan Fruit, The Dannon Company, Dole Food Company, HJ Heinz Company, Harris Teeter Company, Hershey Foods Corporation, Lifelines Technology Inc., McCormick & Company Inc., Nabisco Foods Group, Ocean Spray Cranberries Inc., Procter & Gamble, Quaker Oats Company, Ralston Foods, Sunkist Growers, Vandenbergh Foods and Wawona Frozen Foods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
About this article
Cite this article
Hikmat, F., Appel, L. Effects of the DASH diet on blood pressure in patients with and without metabolic syndrome: results from the DASH trial. J Hum Hypertens 28, 170–175 (2014). https://doi.org/10.1038/jhh.2013.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2013.52
Keywords
This article is cited by
-
Association of a traditional Mediterranean diet and non-Mediterranean dietary scores with all-cause and cause-specific mortality: prospective findings from the Moli-sani Study
European Journal of Nutrition (2021)
-
Dietary contributors to hypertension in adults reviewed
Irish Journal of Medical Science (1971 -) (2015)