Abstract
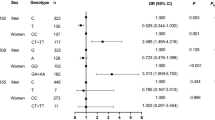
This study examined renin–angiotensin–aldosterone (RAAS) system gene variants for associations with cardiovascular risk factors and outcomes in coronary heart disease. Coronary disease patients (n=1186) were genotyped for 21 single-nucleotide polymorphisms (SNPs) within angiotensinogen (AGT), angiotensin-converting enzyme (ACE), angiotensin-II type-1 receptor (AGTR1) and aldosterone synthase (CYP11B2). Associations with all-cause mortality and cardiovascular readmissions were assessed over a median of 3.0 years. The AGT M235T ‘T’ allele was associated with a younger age of clinical coronary disease onset (P=0.006), and the AGT rs2478545 minor allele was associated with lower circulating natriuretic peptides (P=0.0001–P=0.001) and E/E1 (P=0.018). Minor alleles of AGT SNPs rs1926723 and rs11122576 were associated with more frequent history of renal disease (P⩽0.04) and type-2 diabetes (P⩽0.02), higher body mass index (P⩽0.02) and greater mortality (P⩽0.007). AGT rs11568054 minor allele carriers had more frequent history of renal disease (P=0.04) and higher plasma creatinine (P=0.033). AGT rs6687360 minor allele carriers exhibited worse survival (P=0.02). ACE rs4267385 was associated with older clinical coronary disease onset (P=0.008) and hypertension (P=0.013) onset, increased plasma creatinine (P=0.01), yet greater mortality (P=0.044). Less history of hypertension was observed with the AGTR1 rs12685977 minor allele (P=0.039). Genetic variation within the RAAS was associated with cardiovascular risk factors and accordingly poorer survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Khor GL . Cardiovascular epidemiology in the Asia-Pacific region. Asia Pac J Clin Nutr 2001; 10: 76–80.
O'Toole TE, Conklin DJ, Bhatnagar A . Environmental risk factors for heart disease. Rev Environ Health 2008; 23: 167–202.
Cambien F . Genetics and coronary heart disease. Future Cardiol 2005; 1: 17–27.
Wellcome Trust Case Control Consortium Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Kurdi M, De Mello WC, Booz GW . Working outside the system: an update on the unconventional behavior of the renin-angiotensin system components. Int J Biochem Cell Biol 2005; 37: 1357–1367.
Atlas SA . The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 2007; 13: 9–20.
Lavoie JL, Sigmund CD . Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology 2003; 144: 2179–2183.
Weber KT . Aldosterone in congestive heart failure. N Engl J Med 2001; 345: 1689–1697.
White PC . Disorders of aldosterone biosynthesis and action. N Engl J Med 1994; 331: 250–248.
Schunkert H, Hengstenberg C, Holmer SR, Broeckel U, Luchner A, Muscholl MW et al. Lack of association between a polymorphism of the aldosterone synthase gene and left ventricular structure. Circulation 1999; 99: 2255–2260.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999; 341: 709–717.
Jeunemaitre X . Genetics of the human renin angiotensin system. J Mol Med 2008; 86: 637–641.
Yandle TG, Espiner EA, Nicholls MG, Duff H . Radioimmunoassay and characterization of atrial natriuretic peptide in human plasma. J Clin Endocrinol Metab 1986; 63: 72–79.
Seidler T, Pemberton C, Yandle T, Espiner E, Nicholls G, Richards M . The amino terminal regions of proBNP and proANP oligomerise through leucine zipper-like coiled-coil motifs. Biochem Biophys Res Commun 1999; 255: 495–501.
Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T . Combined neutral endopeptidase and angiotensin-converting enzyme inhibition in heart failure: role of natriuretic peptides and angiotensin II. J Cardiovasc Pharmacol 1998; 31: 116–125.
Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA . Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin Endocrinol 1997; 47: 287–296.
Yandle TG, Fisher S, Charles C, Espiner EA, Richards AM . The ovine hypothalamus and pituitary have markedly different distribution of C-type natriuretic peptide forms. Peptides 1993; 14: 713–716.
Steiner AL, Pagliara AS, Chase LR, Kipnis DM . Radioimmunoassay for cyclic nucleotides. II. Adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in mammalian tissues and body fluids. J Biol Chem 1972; 247: 1114–1120.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367.
Palmer BR, Pilbrow AP, Yandle TG, Frampton CM, Richards AM, Nicholls MG et al. Angiotensin-converting enzyme gene polymorphism interacts with left ventricular ejection fraction and brain natriuretic peptide levels to predict mortality after myocardial infarction. J Am Coll Cardiol 2003; 41: 729–736.
Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ et al. Framingham Heart Study 100 K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet 2007; 8 (Suppl 1): S3.
Pilbrow AP, Palmer BR, Frampton CM, Yandle TG, Troughton RW, Campbell E et al. Angiotensinogen M235T and T174M gene polymorphisms in combination doubles the risk of mortality in heart failure. Hypertension 2007; 49: 322–327.
Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A et al. Molecular basis of human hypertension: role of angiotensinogen. Cell 1992; 71: 169–180.
Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 1997; 99: 1786–1797.
Richards AM, McDonald D, Fitzpatrick MA, Nicholls MG, Espiner EA, Ikram H et al. Atrial natriuretic hormone has biological effects in man at physiological plasma concentrations. J Clin Endocrinol Metab 1988; 67: 1134–1139.
Ribeiro-Oliveira A, Nogueira AI, Pereira RM, Boas WW, Dos Santos RA . Simoes e Silva AC. The renin-angiotensin system and diabetes: an update. Vasc Health Risk Manag 2008; 4: 787–803.
Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 1992; 51: 197–205.
Higaki J, Baba S, Katsuya T, Sato N, Ishikawa K, Mannami T et al. Deletion allele of angiotensin-converting enzyme gene increases risk of essential hypertension in Japanese men: the Suita Study. Circulation 2000; 101: 2060–2065.
Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 1992; 359: 641–644.
Davis GK, Millner RW, Roberts DH . Angiotensin converting enzyme (ACE) gene expression in the human left ventricle: effect of ACE gene insertion/deletion polymorphism and left ventricular function. Eur J Heart Fail 2000; 2: 253–256.
Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension 1994; 24: 63–69.
Castellano M, Muiesan ML, Beschi M, Rizzoni D, Cinelli A, Salvetti M et al. Angiotensin II type 1 receptor A/C1166 polymorphism. Relationships with blood pressure and cardiovascular structure. Hypertension 1996; 28: 1076–1080.
Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3' untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet 2007; 81: 405–413.
Smilde TD, Zuurman MW, Hillege HL, van Veldhuisen DJ, van Gilst WH, van der Steege G et al. Renal function dependent association of AGTR1 polymorphism (A1166C) and electrocardiographic left-ventricular hypertrophy. Am J Hypertens 2007; 20: 1097–1103.
Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE . Differential regulation of aldosterone synthase and 11beta-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol 2002; 28: 125–135.
Sookoian S, Gianotti TF, Gonzalez CD, Pirola CJ . Association of the C-344T aldosterone synthase gene variant with essential hypertension: a meta-analysis. J Hypertens 2007; 25: 5–13.
Patel S, Steeds R, Channer K, Samani NJ . Analysis of promoter region polymorphism in the aldosterone synthase gene (CYP11B2) as a risk factor for myocardial infarction. Am J Hypertens 2000; 13: 134–139.
McNamara DM, Tam SW, Sabolinski ML, Tobelmann P, Janosko K, Taylor AL et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48: 1277–1282.
Acknowledgements
We thank the study participants, the clinical research staff of the Cardioendocrine Research Group (Christchurch) and the Cardiovascular Research Laboratory (Auckland) and are grateful to the assay staff of Cardioendolab, University of Otago, Christchurch. This work was funded by the Health Research Council of New Zealand (Auckland, New Zealand); New Zealand Lotteries Grant Board (Wellington, New Zealand), and National Heart Foundation of New Zealand (Auckland, New Zealand).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Human Hypertension website
Rights and permissions
About this article
Cite this article
Ellis, K., Palmer, B., Frampton, C. et al. Genetic variation in the renin–angiotensin–aldosterone system is associated with cardiovascular risk factors and early mortality in established coronary heart disease. J Hum Hypertens 27, 237–244 (2013). https://doi.org/10.1038/jhh.2012.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2012.24
Keywords
This article is cited by
-
Impact of renin–angiotensin–aldosterone system polymorphisms on myocardial perfusion: Correlations with myocardial single photon emission computed tomography-derived parameters
Journal of Nuclear Cardiology (2019)
-
Plasma levels of soluble VEGF receptor isoforms, circulating pterins and VEGF system SNPs as prognostic biomarkers in patients with acute coronary syndromes
BMC Cardiovascular Disorders (2018)
-
Case–control association study of polymorphisms in the angiotensinogen and angiotensin-converting enzyme genes and coronary artery disease and systemic artery hypertension in African-Brazilians and Caucasian-Brazilians
Journal of Genetics (2016)
-
Humid heat exposure induced oxidative stress and apoptosis in cardiomyocytes through the angiotensin II signaling pathway
Heart and Vessels (2015)
-
Relationship of renin-angiotensin-aldosterone system polymorphisms and phenotypes to mortality in Chinese coronary atherosclerosis patients
Scientific Reports (2014)