Abstract
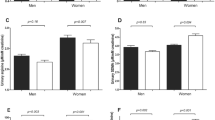
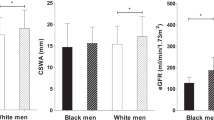
Many mechanisms, including oxidative stress, contribute to hypertension. This study investigated the possible associations between oxidative stress, blood pressure and arterial stiffness in black South Africans. Ambulatory blood pressure measurements were taken for 101 black South African men and 99 women. The stiffness indices included ambulatory arterial stiffness index (AASI) and pulse pressure (PP). Reactive oxygen species (ROS) levels (P<0.0001) were higher in the African women compared with men. ROS levels were also higher in hypertensive compared with normotensive men. The 24 h systolic blood pressure (SBP; P<0.01), 24 h diastolic blood pressure (DBP; P<0.0001) and pulse wave velocity (PWV; P<0.01) were significantly higher in African men compared with women. There were unadjusted positive associations of 24 h SBP (r=0.33; P=0.001), 24 h DBP (r=0.26; P=0.008) and 24 h PP (r=0.29; P=0.003) with ROS in African men only. A positive association between AASI and ROS existed only in hypertensive men (r=0.27; P=0.035), but became nonsignificant (B=0.0014; P=0.14) after adjustments. Adjusted, positive associations of 24 h SBP (B=0.181; P=0.018) and 24 h PP (B=0.086; P=0.050) with ROS were again only evident in African men. ROS is positively associated with SBP and PP in African men, suggesting that increased ROS levels may contribute to hypertension in this population group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Opie LH, Seedat YK . Hypertension in sub-Saharan African populations. Circulation 2005; 112: 3562–3568.
Seedat Y, Seedat M, Hackland D . Prevalence of hypertension in the urban and rural Zulu. Br Med J 1982; 36: 256–261.
Van Rooyen JM, Huisman HW, Eloff FC, Laubscher PJ, Malan L, Steyn HS et al. Cardiovascular reactivity in Black South-African males of different age groups: the influence of urbanization. Ethn Dis 2002; 12: 69–75.
Zalba G, Jose GS, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ et al. Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension 2001; 38: 1395–1399.
Huisman HW, van Rooyen JM, Malan NT, Eloff FC, Malan L, Laubscher PJ et al. Prolactin, testosterone and cortisol as possible markers of changes in cardiovascular function associated with urbanization. J Hum Hypertens 2002; 16: 829–835.
Cai H, Harrison DG . Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840–844.
Zieman SJ, Melenovsky V, Kass DA . Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25: 932–943.
Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG . Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997; 95: 588–593.
Zeiher AM, Drexler H, Saurbier B, Just H . Endothelium-mediated coronary blood flow modulation in humans. J Clin Invest 1993; 92: 652–662.
O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005; 23: 697.
Dolan E, Li Y, Thijs L, McCormack P, Staessen JA, O’Brien E et al. Ambulatory arterial stiffness index: rationale and methodology. Blood Press Monit 2006; 11: 103.
Norton K, Olds T . Anthropometrica: A textbook of body measurements for sports and health courses. Sydney: UNSW Press, 1996.
Yao JK, Reddy R, van Kammen DP . Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res 1998; 80: 29–39.
Herzum I, Rieger T, Funke J . Evaluation of the consolidated Beckman Coulter UniCel (R) DxC 880i analyser: 1714_A. Clin Chem Lab Med 2008; 46: A72.
Cockcroft D, Gault M . Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41.
Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T . High-throughput spectrophotometric assay of reactive oxygen species in serum. Mut Res 2007; 631: 55–61.
Mitrunen K, Sillanpaa P, Kataja V, Eskelinen M, Kosma VM, Benhamou S et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 2001; 22: 827.
Schillaci G, Parati G, Pirro M, Pucci G, Mannarino MR, Sperandini L et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension 2007; 49: 986.
Touyz RM . Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 2004; 44: 248–252.
Vandenbroucke JP, Rosing J, Bloemenkamp KWM, Middeldorp S, Helmerhorst FM, Bouma BN et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med 2001; 344: 1527–1535.
Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005; 111: 3384–3390.
Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ . Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase an in vitro demonstration in human coronary artery endothelial cells. Circulation 2003; 107: 2342–2347.
Fernandez-Checa J, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M et al. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol Gastrointest Liver Physiol 1997; 273: 7–17.
Schutte R, Schutte AE, Huisman HW, van Rooyen JM, Malan NT, Péter S et al. Blood glutathione and subclinical atherosclerosis in African men: the SABPA study. Am J Hypertens 2009; 22: 1154–1159.
Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF et al. PPARá activator effects on ang II-induced vascular oxidative stress and inflammation. Hypertension 2002; 40: 866–871.
Westerhof N, Lankhaar JW, Westerhof BE . Letter to the editor: Ambulatory arterial stiffness index is not a stiffness parameter but a ventriculo-arterial coupling factor. Hypertension 2007; 49: e7.
Acknowledgements
The Sympathetic Activity and Ambulatory Blood Pressure in Africans (SABPA) study would not have been possible without the voluntary collaboration of the participants and the Department of Education, North West Province, South Africa. We gratefully acknowledge the technical assistance of Mrs Tina Scholtz, Mrs C van Deventer and Sr Chrissie Lessing. Research included in the present study was partially funded by the National Research Foundation, South Africa; the North-West University, Potchefstroom, South Africa; and the Metabolic Syndrome Institute, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kruger, R., Schutte, R., Huisman, H. et al. Associations between reactive oxygen species, blood pressure and arterial stiffness in black South Africans: the SABPA study. J Hum Hypertens 26, 91–97 (2012). https://doi.org/10.1038/jhh.2010.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2010.134
Keywords
This article is cited by
-
Nitric oxide-related markers link inversely to blood pressure in black boys and men: the ASOS and African-PREDICT studies
Amino Acids (2020)
-
Three-year change in oxidative stress markers is linked to target organ damage in black and white men: the SABPA study
Hypertension Research (2019)
-
The relation of blood pressure and carotid intima-media thickness with the glutathione cycle in a young bi-ethnic population: the African-PREDICT study
Journal of Human Hypertension (2018)
-
The association of endothelin-1 with markers of oxidative stress in a biethnic South African cohort: the SABPA study
Hypertension Research (2017)
-
Recent advances in understanding hypertension development in sub-Saharan Africa
Journal of Human Hypertension (2017)