Abstract
Mitochondrial trifunctional protein (TFP) deficiency is an inherited metabolic disorder of mitochondrial fatty-acid oxidation. Isolated long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency is often reported in Caucasian countries due to a common mutation. However, the molecular and clinical basis of complete TFP deficiency has not been extensively reported. In this study, 14 Japanese cases (13 families) with complete TFP deficiency, including 9 previously reported cases, were analyzed to clarify the clinical and molecular characteristics of TFP deficiency. The clinical types of the 14 patients were as follows: 12 cases of neonatal (n=7) or myopathic (n=5) types and 2 cases of intermediate type. Peripheral neuropathy was found in four cases and hypocalcemia due to hypoparathyroidism, which is rarely reported in Caucasian patients, had developed in four cases. Maternal hemolysis, elevated liver enzymes and low platelet count syndrome and acute fatty liver of pregnancy were noted in two and one mothers, respectively. Fourteen mutations were identified in 26 alleles in Japanese patients, including two novel mutations (HADHA: c.361C>T, and HADHA-HADHB: g.26233880_ 26248855del), although no common mutations were found. This study suggests that the molecular and clinical aspects of Japanese patients with TFP deficiencies differ from those of Caucasian patients.
Similar content being viewed by others
Introduction
Mitochondrial trifunctional protein (TFP; OMIM: 609015) consists of three enzymes that are involved in the final three steps of β-oxidation of long-chain fatty acids: long-chain enoyl-CoA hydratase (EC 4.2.1.74), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD, EC 1.1.1.211) and long-chain 3-ketoacyl-CoA thiolase (LCKT, EC 2.3.1.16).1 TFP is a hetero-octamer of four α-subunits harboring LCHAD and long-chain enoyl-CoA hydratase as well as four β-subunits harboring LCKT, which are located in the mitochondrial inner membrane. These subunits are encoded by HADHA (OMIM: 600890) and HADHB (OMIM: 143450), respectively, and both share a bidirectional promoter on chromosome 2p23. Mutations in HADHA or HADHB disrupt the conformation of the TFP hetero-octamer, which results in reduced activity of all three enzymes.2, 3, 4, 5 However, a mutation in the catalytic region reduces the activity of only one enzyme without inducing a conformational change. Therefore, mutations in the HADHA and HADHB genes result in two different biochemical phenotypes. Complete TFP deficiency involves the reduced activity of all three TFP enzymes, whereas isolated LCHAD or LCKT deficiencies are associated with a deficiency in the activity of each enzyme.6, 7 The majority of Caucasian patients reported in the literature presented isolated LCHAD deficiency due to a common mutation, c.1528G>C, in the HADHA gene, but few reports have examined complete TFP deficiency in Caucasian patients.8, 9 However, in Japan, only a few patients with complete TFP deficiency have been reported,10, 11, 12, 13, 14, 15 and no patients have been reported with isolated LCHAD deficiency.
Complete TFP deficiency is clinically classified into three types, similar to other fatty-acid oxidation disorders: (1) lethal type (neonatal-onset form), which includes the development of profound hypoglycemia, lactic acidosis and cardiomyopathy during the neonatal period and is associated with a high mortality rate; (2) intermediate type (infant-onset form), which is accompanied by episodic hypoketotic hypoglycemia or hepatic dysfunction following infection or long periods of fasting during the infancy period; and (3) myopathic type (adult-onset form), which is characterized by muscular symptoms, such as intermittent myalgia or rhabdomyolysis, and is associated with prolonged exercise in adolescence or adulthood.10 Complete TFP deficiency or isolated LCHAD deficiency likely present unique clinical symptoms, such as retinopathy or neuropathy.16, 17 Moreover, pregnant women with a fetus affected with complete TFP deficiency or isolated LCHAD deficiency often show hemolysis, elevated liver enzymes and low platelet count syndrome or acute fatty liver of pregnancy.7, 18, 19
To identify the clinical and genetic differences between patients of European and Japanese descent, we investigated the clinical and molecular characteristics of 14 Japanese patients with complete TFP deficiency.
Materials and methods
The Ethical Committee of Shimane University Faculty of Medicine approved the study protocol, and the parents of the participants provided written informed consent.
Participants
Fourteen Japanese cases (13 families) with complete TFP deficiency were diagnosed at Shimane University. In addition to acylcarnitine and genetic analyses, western blots, enzyme assays, or both were conducted for 12 of the 14 cases. Five cases had previously been reported,10 four cases were described in case reports,11, 13, 15 and five cases were newly diagnosed and analyzed. The clinical course, birth records, present status and therapeutic regimens were obtained using questionnaires administered by the attending physicians.
Genetic analysis
Genomic DNA was extracted from fibroblasts or lymphoblastoid cells using the QIAamp DNA Micro Kit (Qiagen GmbH, Hilden, Germany). Both the HADHA and HADHB genes, which encode TFP, were sequenced as previously reported.10 Genetic structures were obtained from the GenBank database.
Western blots
A western blot analysis of TFP in cultured fibroblasts or lymphoblastoid cells was performed using a rabbit polyclonal antibody raised against both the α- and β-subunits of TFP; Dr T Hashimoto, Professor Emeritus, Shinshu University, Matsumoto, Japan kindly provided the antibody. The signals of the α- and β-subunits were visualized using an ImmunoPure NBT/BCIP Substrate Kit (Promega, Madison, WI, USA) as previously described.20
Enzyme assay
LCHAD and LKAT activities were determined in fibroblasts or lymphoblastoid cells, and the values of LCHAD and LCKT activity are expressed as % of normal control (means±s.d.). Control data represent individuals without fatty-acid oxidation disorders (n=5, 1: fibroblasts and lymphoblastoid cell, respectively).
As described in a previous report,10 LCKT activity was measured in 100 mM Tris-HCl (pH 8.3), 50 mM KCl, 25 mM MgCl2, 0.1% (w/v) Triton X-100 and 0.2 mg ml−1 bovine serum albumin, with 10 μM 2-ketopalmitoyl-CoA as a substrate. The reaction was started by the addition of coenzyme-A, followed by absorbance measurements at 303 nm using a Shimadzu UV-1600 Spectrometer (Shimadzu, Kyoto, Japan).
As described in previous reports,21, 22 the activity of LCHAD in the fibroblast homogenates was measured in a medium containing 50 mmol 2-(N-morpholino)-ethanesulphonic acid, 100 mmol potassium phosphate, 0.1 mmol dithiothreitol, 0.1% (w/v) Triton X-100, 100 mmol NADH (final pH 6.16) with or without a final concentration of 5 mmol l−1 N-ethylmaleimide to inhibit LCHAD activity. The reaction was initiated by adding 3-ketopalmitoyl-CoA at a final concentration of 25 μmol l−1, and absorbance was measured at 340 nm.
Results
Clinical manifestation
The clinical features and courses of the 14 cases (from 13 families) with complete TFP deficiency, including the previously reported cases, are summarized in Table 1. All patients were diagnosed via high-risk screening and not newborn mass screening. Cases 10 and 11 were siblings. Three of the 13 families (cases 7, 10–11 and 14) demonstrated consanguineous marriage.
Seven of the 14 cases (50%) were diagnosed as the lethal type, all of whom presented with severe symptoms including metabolic acidosis, cardiomyopathy and respiratory failure within 8 days of life. Six of the seven cases (cases 1–5, 7) died soon after birth (between day 6 and month 3), despite various treatments. The remaining case (case 6) was delivered via a scheduled Cesarean section and suddenly developed cardiopulmonary arrest in the hospital after 45 h of life. Cardiac pulmonary resuscitation was performed successfully for approximately fifty minutes and he is now 3 years old. Two episodes of rhabdomyolysis (creatine kinase >100 000 U l−1) have been noted following infectious disease.
Only two of the 14 cases (14%) that had been previously reported were classified as the intermediate type. Case 8 presented unconsciousness with lactic acidosis at 9 months of age. After that episode, she developed recurrent rhabdomyolysis. Case 9 initially developed respiratory failure and hypotonia at 13 months. These two cases did not present with recurrent hypoketotic hypoglycemia.
Five of the 14 cases (36%) were classified as the myopathic type. Cases 10 and 11 needed assistance due to artificial ventilation and a wheelchair, respectively, whereas the other three patients presented with relatively milder muscular symptoms, such as rhabdomyolysis, myalgia or muscular hypotonia following infection. In addition, the age at onset of four cases ranged from 2 to 5 years, and recurrent rhabdomyolysis developed a few years later.
Although complications due to retinopathy were not present in any of the cases, peripheral neuropathy of motor dysfunction and abnormal sensory nerves occurred in four cases (cases 10–12 and 14). Two of the four cases with peripheral neuropathy (cases 10 and 11) had been misdiagnosed with CMT (Charcot-Marie-Tooth) disease. Moreover, cases 6, 9, 10 and 11 were associated with hypocalcemia due to hypoparathyroidism. Two of the pregnancies were complicated by hemolysis, elevated liver enzymes and low platelet count syndrome (cases 3 and 8), and one was complicated by acute fatty liver of pregnancy (case 5).
In addition to the usual therapeutic recommendation of fatty-acid oxidation disorders (FAODs), bezafibrate (BEZ) was administered to five cases presenting with recurrent rhabdomyolysis (cases 6, 8, 12, 13 and 14). Significant reductions in the number of rhabdomyolysis episodes were observed in cases 6, 8 and 13, whereas no changes were detected in cases 12 and 14 according to the attending physicians.
Western blotting
Figure 1 shows the western blots for TFP performed on the fibroblasts of newly diagnosed cases (case 1, 5, 6 and 13), whereas other patients, with assays performed at different times (case 4, 7–12), had been reported. No TFP-α or -β proteins were visualized in any of the cases, whereas the presence of very long-chain acyl-CoA dehydrogenase as a positive control was confirmed. Eventually, 12 cases were diagnosed with complete TFP deficiency.
Western blot analysis of fibroblasts in patients with newly diagnosed complete TFP deficiency (case 1, 5, 6 and 13). Proteins from the indicated sources were examined. TFPα and TFPβ represent alpha- and beta-subunits of TFP, respectively. The positions of the alpha- and beta-subunits of the TFP and VLCAD proteins are indicated by arrows. VLCAD: very long-chain acyl-CoA dehydrogenase.
The enzyme activities of LCHAD and LCKT
The LCHAD and LCKT activities of fibroblasts (cases 1, 4–9 and 12–14) and lymphoblastoid cells (cases 10 and 11) were also measured (see Table 2).
In normal control, the LCHAD and LCKT activity of fibroblast was 68.3±14.2 nmol min−1 mg−1 protein, 6.5±1.0 nmol min−1 mg−1 protein (mean±s.d.), respectively. The LCHAD and LCKT activity of lymphoblastoid cell was 73.3 nmol min−1 mg−1 protein, 8.0 nmol min−1 mg−1 protein, respectively.
Compared with the normal control, a reduction of LCKT activity was observed in all 12 cases (13.7±5.2%, 8.4±5.0%: using fibroblasts and lymphoblastoid cells, respectively). The activity of LCHAD remained relatively high (27.8±6.6%, 48.1±1.1%: using fibroblasts and lymphoblastoid cells, respectively). N-ethylmaleimide, a specific inhibitor of LCHAD, obviously inhibited the LCHAD activities in the controls, whereas those in the patients remained almost unchanged, indicating that N-ethylmaleimide could not further inhibit the LCHAD activity of patients. These high residual activity of LCHAD was likely derived from short-chain hydroxyacyl-coA dehydrogenase, which was not inhibited by N-ethylmaleimide in previous reports.21 Eventually, both the reductions of LCHAD and LCKT activities demonstrated that 12 patients suffered from complete TFP deficiency and not from isolated LCHAD or LCKT deficiency.
Genetic analysis
The identified mutations of the 13 cases are summarized in Table 2. Mutations in three of the 13 cases were identified on HADHA, and those of the remaining 10 cases were identified on HADHB. Direct sequencing following diagnosis by enzymatic assay and western blotting failed to identify mutations in the coding regions or exon–intron boundaries of HADHA and HADHB in case 13. In this study, two novel mutations were identified: HADHA: c.361C>T, and HADHA-HAHDB: g.26233880_ 26248855del. These novel mutations were not detected in the 100 alleles from unaffected Japanese individuals.
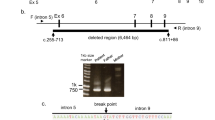
In case 3, a homozygote of c.157C>T on HADHA was initially suspected via direct sequencing; however, a maternal molecular analysis could not identify this mutation. The large deletion of HADHA was suspected based on the complementary DNA analysis. Eventually, long-PCR sequencing revealed a 14 976 bp deletion spanning from intron 5 of HADHA to intron 1 of HADHB owing to Alu arrangements (Figure 2).
Discussion
This study clearly demonstrated the following clinical and molecular characteristics of Japanese patients with TFP deficiency, compared with Caucasian patients previously reported (Table 3): (1) the lethal and myopathic types predominated, whereas the intermediate type was small and (2) despite the large numbers of mutations that were identified on HADHB, no common mutations were detected in Japanese patients.
Most of the patients with the lethal type died soon after birth, suggesting that this type of complete TFP deficiency has a severe prognosis. The acylcarnitine levels in the amniotic fluid from a fetus with a TFP deficiency were previously reported to be elevated, and those with TFP deficiencies developed intrauterine cardiomyopathy owing to cardiac energy deficiency,20, 23 suggesting that TFP has an important role, even during the neonatal period. Therefore, any intensive therapies after birth cannot save patients with the lethal type of complete TFP deficiency.
Our data and a previous report16 found that only 13–14% of patients had the intermediate type of complete TFP deficiency. Although Boutron et al.9 reported that hypoketotic hypoglycemia was observed in 67% of patients, their main objective was to examine patients with isolated LCHAD deficiency (Table 3). Hypoketotic hypoglycemia, a typical symptom of the intermediate type, was not common in Japanese or Caucasian patients with complete TFP deficiency.
All five patients with the myopathic type displayed a relatively positive prognosis. However, the muscular symptoms of these cases (except for case 14) were recognized at an earlier age than in the myopathic type of the other FAODs, which was also reported among Caucasians with complete TFP deficiency.16, 24 Moreover, in previous reports, patients with complete TFP deficiency showed prominent MRI signal intensity changes on T1-weighted and short tau inversion recovery sequences from the girdle to lower leg among FAODs.25 Earlier onset and prominent abnormal MRI findings may reflect the severity of TFP deficiency compared with the other FAODs.
We did not observe retinopathy in this study, whereas peripheral neuropathy was present in four of 14 patients (29%). Similarly, in previous reports of Caucasian patients, retinopathy (12%) was a less-common complication than peripheral neuropathy (53–79%).8, 26 According to previous reports,17 patients with complete TFP deficiency show a milder accumulation of toxic 3-OH-acylcarnitines than those with isolated LCHAD deficiency, which may be associated with the lower morbidity rate of retinopathy. Interestingly, two of the four cases with peripheral neuropathy had been misdiagnosed with CMT disease as previously reported.13, 16, 27 These findings indicated that patients with neurological symptoms of CMT diseases at an early age should be carefully reexamined for underlying TFP deficiency. Complications of hypoparathyroidism were identified in four cases (cases 6, 9, 10 and 11), three of whom (case 6, 10 and 11) had c.1175C>T in at least one allele (homozygotes in two and a compound heterozygote in one case). Few reports have described hypoparathyroidism in Caucasian patients with TFP deficiency or other FAODs.26, 28 These findings suggest that hypoparathyroidism is a unique characteristic of Japanese patients with complete TFP deficiency, especially in those with the c.1175C>T mutation. Three pregnant women suffered from hemolysis, elevated liver enzymes and low platelet count syndrome or acute fatty liver of pregnancy, including two cases of the lethal type and one case of the intermediate type. The morbidity rate of the present study was 21% (three of the 14 cases), which is relatively lower than the rate for those with isolated LCHAD deficiency (14–78%).7, 18, 19 However, pregnant women with fetuses with complete TFP deficiency required monitoring of maternal liver dysfunction.
Notably, BEZ demonstrated clinical improvements, such as the reduction of rhabdomyolysis, only in three of five cases, suggesting that BEZ can be used to treat muscular symptoms in specific cases with TFP deficiency. To date, the effect of BEZ on FAODs has been controversial. A randomized controlled trial did not demonstrate efficacy improvements in fatty-acid oxidation flux (FAO) and heart rate during exercise in adults with deficiencies in carnitine palmitoyltranferase-2 and very long-chain acyl-CoA dehydrogenase,29 whereas improvements of the acylcarnitine profile, FAO and enzyme activities were reported in vitro.30, 31 Our limited data could not determine the cause of these different levels of effectiveness of BEZ. Additional studies using larger sample sizes under the same conditions are required.
This study identified the first Japanese patients with TFP deficiency caused by a mutation in HADHA. All three Japanese patients with mutations in HADHA exhibited the lethal type, although each HADHA or HADHB mutation was assumed to have the same effect on TFP deficiency.2 This occurred because mutations such as splicing inhibition, nonsense mutations or large deletions (which can lead to the complete elimination of TFP activity) were present on HADHA rather than HADHB. A large novel deletion was found in Case 3 spanning from HADHA to HADHB. Although one large deletion on HADHB was previously reported,9 a large deletion spanning from HADHA to HADHB has not been reported previously. The most common mutation in the present study was c.1331G>A in HADHB, which was found in three families. Mutations in HADHB are more frequent in Japanese cases with complete TFP deficiency but have been found to be heterogeneous. Similarly, mutations in HADHB have been predominantly found in East Asian participants from countries such as Korea or China;27, 32, 33, 34 thus, this pattern may be specific to East Asian ethnicities. In contrast, as in previous reports, HADHA mutations are more common9 or equal to HADHB mutations in Caucasian countries.10, 16
This study analyzed the clinical and molecular features of Japanese patients with complete TFP deficiency. In Japan, mutations were predominantly found in HADHB, in contrast to Caucasian patients. The clinical type was primarily categorized into either the lethal or myopathic type, and the age at onset tended to be earlier than that of other FAODs. Hypoparathyroidism and peripheral neuropathy similar to CMT might be characteristic of the Japanese population. Maternal hemolysis, elevated liver enzymes and low platelet count syndrome, acute fatty liver of pregnancy, or both may be observed in a pregnancy with a TFP-deficient fetus.
References
Uchida, Y., Izai, K., Orii, T. & Hashimoto, T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J. Biol. Chem. 267, 1034–1041 (1992).
Spiekerkoetter, U., Khuchua, Z., Yue, Z., Bennett, M. J. & Strauss, A. W. General mitochondrial trifunctional protein (TFP) deficiency as a result of either alpha- or beta-subunit mutations exhibits similar phenotypes because mutations in either subunit alter TFP complex expression and subunit turnover. Pediatr. Res. 55, 190–196 (2004).
Ushikubo, S., Aoyama, T., Kamijo, T., Wanders, R. J., Rinaldo, P., Vockley, J. et al. Molecular characterization of mitochondrial trifunctional protein deficiency: formation of the enzyme complex is important for stabilization of both alpha- and beta-subunits. Am. J. Hum. Genet. 58, 979–988 (1996).
Yang, B. Z., Heng, H. H., Ding, J. H. & Roe, C. R. The genes for the alpha and beta subunits of the mitochondrial trifunctional protein are both located in the same region of human chromosome 2p23. Genomics 37, 141–143 (1996).
Orii, K. E., Aoyama, T., Souri, M., Jiang, L. L., Orii, K. O., Hayashi, S. et al. Formation of the enzyme complex in mitochondria is required for function of trifunctional beta-oxidation protein. Biochem. Biophys. Res. Commun. 219, 773–777 (1996).
Das, A. M., Illsinger, S., Lucke, T., Hartmann, H., Ruiter, J. P., Steuerwald, U. et al. Isolated mitochondrial long-chain ketoacyl-CoA thiolase deficiency resulting from mutations in the HADHB gene. Clin. Chem. 52, 530–534 (2006).
den Boer, M. E., Wanders, R. J., Morris, A. A., IJlst, L., Heymans, H. S. & Wijburg, F. A. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics 109, 99–104 (2002).
Spiekerkoetter, U., Sun, B., Khuchua, Z., Bennett, M. J. & Strauss, A. W. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 21, 598–607 (2003).
Boutron, A., Acquaviva, C., Vianey-Saban, C., de Lonlay, P., de Baulny, H. O., Guffon, N. et al. Comprehensive cDNA study and quantitative analysis of mutant HADHA and HADHB transcripts in a French cohort of 52 patients with mitochondrial trifunctional protein deficiency. Mol. Genet. Metab. 103, 341–348 (2011).
Purevsuren, J., Fukao, T., Hasegawa, Y., Kobayashi, H., Li, H., Mushimoto, Y. et al. Clinical and molecular aspects of Japanese patients with mitochondrial trifunctional protein deficiency. Mol. Genet. Metab. 98, 372–377 (2009).
Yagi, M., Lee, T., Awano, H., Tsuji, M., Tajima, G., Kobayashi, H. et al. A patient with mitochondrial trifunctional protein deficiency due to the mutations in the HADHB gene showed recurrent myalgia since early childhood and was diagnosed in adolescence. Mol. Genet. Metab. 104, 556–559 (2011).
Purevsuren, J., Fukao, T., Hasegawa, Y., Fukuda, S., Kobayashi, H. & Yamaguchi, S. Study of deep intronic sequence exonization in a Japanese neonate with a mitochondrial trifunctional protein deficiency. Mol. Genet. Metab. 95, 46–51 (2008).
Naiki, M., Ochi, N., Kato, Y. S., Purevsuren, J., Yamada, K., Kimura, R. et al. Mutations in HADHB, which encodes the beta-subunit of mitochondrial trifunctional protein, cause infantile onset hypoparathyroidism and peripheral polyneuropathy. Am. J. Med. Genet. A 164, 1180–1187 (2014).
Orii, K. E., Aoyama, T., Wakui, K., Fukushima, Y., Miyajima, H., Yamaguchi, S. et al. Genomic and mutational analysis of the mitochondrial trifunctional protein beta-subunit (HADHB) gene in patients with trifunctional protein deficiency. Hum. Mol. Genet. 6, 1215–1224 (1997).
Kobayashi, T., Minami, S., Mitani, A., Tanizaki, Y., Booka, M., Okutani, T. et al. Acute fatty liver of pregnancy associated with fetal mitochondrial trifunctional protein deficiency. J. Obstet. Gynaecol. Res. 41, 799–802 (2015).
Spiekerkoetter, U., Bennett, M. J., Ben-Zeev, B., Strauss, A. W. & Tein, I. Peripheral neuropathy, episodic myoglobinuria, and respiratory failure in deficiency of the mitochondrial trifunctional protein. Muscle Nerve 29, 66–72 (2004).
Fletcher, A. L., Pennesi, M. E., Harding, C. O., Weleber, R. G. & Gillingham, M. B. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol. Genet. Metab. 106, 18–24 (2013).
Tyni, T., Ekholm, E. & Pihko, H. Pregnancy complications are frequent in long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Am. J. Obstet. Gynecol. 178, 603–608 (1998).
Ibdah, J. A., Bennett, M. J., Rinaldo, P., Zhao, Y., Gibson, B., Sims, H. F. et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N. Engl. J. Med. 340, 1723–1731 (1999).
Bo, R., Hasegawa, Y., Yamada, K., Kobayashi, H., Taketani, T., Fukuda, S. et al. A fetus with mitochondrial trifunctional protein deficiency: elevation of 3-OH-acylcarnitines in amniotic fluid functionally assured the genetic diagnosis. Mol. Genet. Metab. Rep. 6, 1–4 (2016).
Wanders, R. J., Ruiter, J. P., IJLst, L., Waterham, H. R. & Houten, S. M. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J. Inherit. Metab. Dis. 33, 479–494 (2010).
Wanders, R. J., IJLst, L., van Gennip, A. H., Jakobs, C., de Jager, J. P., Dorland, L. et al. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 13, 311–314 (1990).
Spierkerkoetter, U., Khuchua, Z., Yue, Z. & Strauss, A. W. The early-onset phenotype of mitochondrial trifunctional protein deficiency: a lethal disorder with multiple tissue involvement. J. Inherit. Metab. Dis. 27, 294–296 (2004).
Olpin, S. E., Clark, S., Andresen, B. S., Bischoff, C., Olsen, R. K., Gregersen, N. et al. Biochemical, clinical and molecular findings in LCHAD and general mitochondrial trifunctional protein deficiency. J. Inherit. Metab. Dis. 28, 533–544 (2005).
Diekman, E. F., van der Pol, W. L., Nievelstein, R. A., Houten, S. M., Wijburg, F. A. & Visser, G. Muscle MRI in patients with long-chain fatty acid oxidation disorders. J. Inherit. Metab. Dis. 37, 405–413 (2014).
den Boer, M. E., Dionisi-Vici, C., Chakrapani, A., van Thuijl, A. O., Wanders, R. J., Wijburg, F. A. et al. Mitochondrial trifunctional protein deficiency: a severe fatty acid oxidation disorder with cardiac and neurologic involvement. J. Pediatr. 142, 684–689 (2003).
Hong, Y. B., Lee, J. H., Park, J. M., Choi, Y. R., Hyun, Y. S., Yoon, B. R. et al. A compound heterozygous mutation in HADHB gene causes an axonal Charcot-Marie-tooth disease. BMC Med. Genet. 14, 125 (2013).
Tyni, T., Rapola, J., Palotie, A. & Pihko, H. Hypoparathyroidism in a patient with long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency caused by the G1528C mutation. J. Pediatr. 131, 766–768 (1997).
Orngreen, M. C., Madsen, K. L., Preisler, N., Andersen, G., Vissing, J. & Laforet, P. Bezafibrate in skeletal muscle fatty acid oxidation disorders: a randomized clinical trial. Neurology 82, 607–613 (2014).
Yamaguchi, S., Li, H., Purevsuren, J., Yamada, K., Furui, M., Takahashi, T. et al. Bezafibrate can be a new treatment option for mitochondrial fatty acid oxidation disorders: evaluation by in vitro probe acylcarnitine assay. Mol. Genet. Metab. 107, 87–91 (2012).
Djouadi, F., Habarou, F., Le Bachelier, C., Ferdinandusse, S., Schlemmer, D., Benoist, J. F. et al. Mitochondrial trifunctional protein deficiency in human cultured fibroblasts: effects of bezafibrate. J. Inherit. Metab. Dis. 39, 47–58 (2016).
Choi, J. H., Yoon, H. R., Kim, G. H., Park, S. J., Shin, Y. L. & Yoo, H. W. Identification of novel mutations of the HADHA and HADHB genes in patients with mitochondrial trifunctional protein deficiency. Int. J. Mol. Med. 19, 81–87 (2007).
Fu, X., Zheng, F., Zhang, Y., Bao, X., Wang, S., Yang, Y. et al. Mitochondrial trifunctional protein deficiency due to HADHB gene mutation in a Chinese family. Mol. Genet. Metab. Rep. 5, 80–84 (2015).
Park, H. D., Kim, S. R., Ki, C. S., Lee, S. Y., Chang, Y. S., Jin, D. K. et al. Two novel HADHB gene mutations in a Korean patient with mitochondrial trifunctional protein deficiency. Ann. Clin. Lab. Sci. 39, 399–404 (2009).
Acknowledgements
We thank Ms M Furui, N Tomita, Y Ito, T Esumi and E Mizuno from the Department of Pediatrics at Shimane University for their technical assistance. Furthermore, we greatly appreciate the attending physicians in charge of the present cases for providing clinical information. Finally, we thank Dr Takashi Hashimoto, Professor Emeritus of Shinshu University, Japan for kindly providing the TFP antibody and 3-ketopalmitoyl-CoA for the enzymatic assay. This research was partially supported by the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development, AMED and Grants from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bo, R., Yamada, K., Kobayashi, H. et al. Clinical and molecular investigation of 14 Japanese patients with complete TFP deficiency: a comparison with Caucasian cases. J Hum Genet 62, 809–814 (2017). https://doi.org/10.1038/jhg.2017.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2017.52
This article is cited by
-
Novel HADHB mutations in a patient with mitochondrial trifunctional protein deficiency
Human Genome Variation (2020)
-
Expanding the genotype–phenotype correlation of childhood sensory polyneuropathy of genetic origin
Scientific Reports (2020)
-
Evaluation of earlier versus later dietary management in long-chain 3-hydroxyacyl-CoA dehydrogenase or mitochondrial trifunctional protein deficiency: a systematic review
Orphanet Journal of Rare Diseases (2019)
-
Management and diagnosis of mitochondrial fatty acid oxidation disorders: focus on very-long-chain acyl-CoA dehydrogenase deficiency
Journal of Human Genetics (2019)