Abstract
Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2), also known as platelet-activating factor (PAF) acetylhydrolase (PAF-AH), is a member of the serine-dependent class of A2 phospholipases that hydrolyze sn2-ester bonds of fragmented or oxidized phospholipids at sites where atherosclerotic plaques are forming. Most circulating Lp-PLA2 is bound to low-density lipoprotein (LDL) particles in plasma and the rest to high-density lipoprotein (HDL). Deficiency of Lp-PLA2 is a predisposing factor for cardiovascular diseases in the Japanese population. We describe here two novel mutations of the gene encoding Lp-PLA2, InsA191 and I317N in Japanese subjects. The first patient, with partial Lp-PLA2 deficiency, was heterozygous for the InsA191 mutation; macrophages from this patient secreted only half the normal amount of Lp-PLA2 in vitro. The other patient, who showed complete Lp-PLA2 deficiency, was a compound heterozygote for the novel I317N mutation and a common V279F mutation; macrophages from that patient failed to secrete any Lp-PLA2. Measurement of Lp-PLA2 mass, activity and Western blotting verified impaired production and secretion of the enzyme after transfection of mutant construct into COS-7 cells. These results indicated that both novel mutants, InsA191 and I317N, impair function of the Lp-PLA2 gene.
Similar content being viewed by others
Introduction
Recent research on atherosclerosis has been focusing on mechanisms involved in formation of atherosclerotic plaques. It is now understood that oxidized low-density lipoprotein (LDL) is a trigger for atherosclerotic lesions; it releases several biologically active compounds, such as fragmented oxidized phospholipids, at sites where new plaques form (Caslake and Packard 2003).
As a class, A2 phospholipases (PLA2) regulate phospholipid content and distribution of LDL and high-density lipoprotein (HDL) in plasma. Lipoprotein-associated PLA2 (Lp-PLA2), a serine-dependent enzyme, hydrolyzes sn2-ester bonds on phospholipids when these molecules are fragmented or oxidized (Stremler et al. 1991; Stafforini et al. 1997). Lp-PLA2, encoded by a gene located at 6p12–p21.1, is a 45.4-kDa (441 amino acids) protein expressed in and secreted by monocytes and macrophages (Stafforini et al. 1996a). About 80% of the Lp-PLA2 in plasma is bound to LDL via specific interaction between the C-terminus of apoB-100, the major constitutive protein of the LDL particle, and two domains of Lp-PLA2, one around tyrosine 205 and the other around tryptophan 115–leucine 116. The rest of the plasma Lp-PLA2 is bound to HDL and VLDL (Stafforini et al. 1999; Kujiraoka et al. 2003). The Lp-PLA2 enzyme is also known as platelet-activating factor (PAF) acetylhydrolase (PAF-AH), since one of its known functions is to deactivate PAF (Stafforini et al. 1987a,b). PAF is a potent lipid mediator of inflammatory disease (Snyder 1995).
A view has developed that Lp-PLA2 may have a protective role in atherosclerosis. For instance, this enzyme degrades atherogenic oxidized lipids (Steinbrecher and Pritchard 1989; Stremler et al. 1989; Stafforini et al. 1992), and deficiency of Lp-PLA2 is a predisposing factor for cardiovascular diseases in people of Japanese ethnicity. That predisposition is mainly due to the presence of a common mutant allele, V279F; homozygotes of the mutant allele comprise 0.04 of this population, although V279F has not been reported in Caucasians (Miwa et al. 1988; Stafforini et al. 1996b). Deficiencies of Lp-PLA2 in Japanese are associated with increased risk of stroke (Hiramoto et al. 1997), coronary artery disease (Yamada et al. 1998, 2000), atherosclerotic occlusive disease (Unno et al. 2000), and abdominal aortic aneurysms (Unno et al. 2002).
We describe here two novel mutant alleles of the Lp-PLA2 gene identified in Japanese patients with Lp-PLA2 deficiency and provide experimental evidence that both cause functional defects.
Materials and methods
Patients and clinical profiles
Patients with complete or partial Lp-PLA2 deficiencies were identified from a group of 295 individuals who were followed clinically for hyperlipidemia or diabetes or were receiving health checkups at the outpatient clinic of the Hokkaido Hospital for Social Insurance (Sapporo, Japan). Blood samples were collected after 12–16 h of fasting (Fujita et al. 2003), and all participants provided informed consent for participation in the study. Measurement of serum total cholesterol (TC), HDL, triglycerides (TG), and apolipoproteins was carried out as described previously (Ishii et al. 2002; Takada et al. 2002). LDL cholesterol was calculated according to the Friedewald formula (Friedewald et al. 1972). Plasma Lp-PLA2 (PAF-AH) activity was determined using [3H] PAF, and Lp-PLA2 mass concentration was determined by sandwich enzyme-linked immunosorbent assay (ELISA) as described by Kujiraoka et al. (2003). Genomic DNA was extracted as previously described (Yoshida et al. 2002). Of the 120 subjects exhibiting Lp-PLA2 deficiency, 119 were found to carry a mutant allele of Lp-PLA2 that is common in Japan—V279F (Kujiraoka et al. 2003).
Two subjects displayed discordance between Lp-PLA2 deficiency status and V279F genotype (Table 1). Case 1, a male patient who was 65 years old, 170 cm tall, and 64 kg in weight, had developed type 2 diabetes (NIDDM) and hypertension at age 62; his elder brother had cerebrovascular disease. The second subject (case 2), also male, was 53 years old, 159 cm tall, and 61 kg in weight; he had hyperlipidemia, hypertension, and cardiovascular disease with an occlusive left-coronary lesion indicated by angiography. His mother had died of acute myocardial infarction at age 68 and his father of cancer at 72.
Isolation and culture of monocyte-derived macrophages
Fresh blood from both subjects was collected in heparin-containing tubes. After mononuclear cells were isolated by centrifugation with Separate L (Muto Pure Chemicals Co., Osaka, Japan), monocyte-derived macrophages were cultured as previously described (Kosaka et al. 2001). On day 7, the medium was replaced with RPMI supplemented with 10% bovine serum albumin, 100 U/ml penicillin, and 100 μg/ml streptomycin. After culture for 2 days or more, the monocyte-derived macrophages and the culture media were separated and reserved for subsequent experiments.
PCR amplification, DNA sequencing, and PCR-RFLP assay
All 12 exons of the Lp-PLA2 gene were individually amplified by PCR under conditions described previously (Iida et al. 2002) using as primers specific oligonucleotides derived from intronic sequences flanking each exon. Sequences surrounding the variations, as well as primer sequences and experimental conditions, were obtained from published reports (Haga et al. 2002; BLAST Accession number 16173643). Each segment was amplified and sequenced according to procedures described previously (Hattori et al. 2002). V279F and Q281R genotypes of Lp-PLA2 were examined in these patients by Invader assay as previously described (Nagano et al. 2002; Saito et al. 2002) using PCR products of the flanking sequences and probes designed and synthesized by the supplier (Ohnishi et al. 2001).
PCR-based assay for the InsA191 and I317N mutations
PCR-RFLP assays for InsA191 and I317N mutant alleles were carried out by amplifying a 283-bp fragment of exon 3 and a 295-bp fragment of exon 10, respectively, using primers 5′-GAAGGCCAGAAACTATGGGAA-3′ (sense) and 5′-GCGACAGACAATATTAGAGTATCAGG-3′ (antisense) for InsA191 and 5′-CCTCAATGTTGGCTAGTATGTAACC-3′ (sense) and 5′-TCGATTGATATTTACCCAAGCTTCA-3′ (antisense) for I317N, followed by digestion with restriction enzyme Tru9I (New England Biolabs, Beverly, MA, USA) at 37°C for 3 h. Digested samples were electrophoresed on a 7.5% PAGE gel, and the bands were visualized with ethIdium bromide.
Site-directed mutagenesis and transfection of COS-7 cells for V279F, Q281R, and I317N mutant alleles
A cDNA fragment encoding amino acids 42–441 of Lp-PLA2 was obtained by RT-PCR from mRNA expressed by peripheral monocyte-derived macrophages and subcloned into the mammalian expression vector pcDNA3.1(+) (Invitrogen, CA, USA) in the manner described by Kujiraoka et al. (2003). Mutant cDNAs (V279F, Q281R, and I317N) were generated from the pcDNA3.1(+)/Lp-PLA2 vector using QuickChange XL Site-Directed Mutagenesis Kits (Stratagene) according to the manufacturer’s instructions. The integrity of each plasmid was verified by DNA sequencing.
One day before transfection, COS-7 cells (4×105 cells/well) were plated onto 6-well plates (Iwaki Glass, Tokyo, Japan) pre-coated with type I collagen (Nagano et al. 2002). The next day, 1 μg of each plasmid was transfected by means of LIPOFECTAMIN 2000 reagent (Invitrogen) into cultures showing 95% confluence. Cells were collected after 48 h of growth in the same medium, and Lp-PLA2 concentrations in the media were determined by ELISA.
Western-blot analysis
Media from the secretion experiments were incubated for 18 h at 4°C with antihuman Lp-PLA2 monoclonal antibody A7G-coated or 8B1-coated Dynabeads (M-450 Sheep anti-mouse IgG; Dynal) at 1 ml medium/1×107 beads. After incubation, the beads were precipitated by centrifugation and washed three times with 0.1% Tween20 (Nakalai Tesque) containing PBS, resuspended in SDS-PAGE sample buffer, heated to 95°C for 5 min, and then subjected to SDS-PAGE. After electrophoresis, the proteins were transferred to PVDF membranes (Millipore), and Western blotting was performed using a polyclonal antibody that had been raised in rabbits against recombinant human Lp-PLA2 expressed by E. coli (Kujiraoka et al. 2003), and goat HRP antirabbit IgG (Zymed). Detection was achieved by means of Western Lightning Chemiluminescence Reagent (PerkinElmer) according to manufacturer recommendations.
Results
Screening patients for Lp-PLA2 deficiency
We examined Lp-PLA2 concentrations in plasma of 295 individuals at the Hokkaido Hospital for Social Insurance using a highly sensitive sandwich ELISA assay that we had developed recently. We also used the Invader assay to screen these patients for a mutant genotype of the Lp-PLA2 gene, V279F, that is common in the Japanese population. Plasma Lp-PLA2 concentrations among heterozygotes for the V279F mutation (n=36) were about half the amounts for wild-type homozygotes (0.79±0.24 μg/ml versus 1.61±0.44 μg/ml, respectively). Subjects who were homozygous for the V279F mutation (n=2) displayed no Lp-PLA2 mass in plasma. Another previously known but rare mutation, Q281R, was present in some patients with partial Lp-PLA2 deficiency (n=4), i.e., who exhibited concentrations of plasma Lp-PLA2 that were reduced by about half (0.83±0.15 μg/ml). However, one subject (case 1) showed reduced Lp-PLA2 mass (0.58 μg/ml) without any known mutations, and another (case 2) had no detectable Lp-PLA2 mass in plasma but was only heterozygous for the V279F mutation (Table 1).
Detection of novel InsA191 and I317N mutations of Lp-PLA2
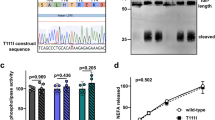
Screening the Lp-PLA2 gene for mutations in case 1 identified heterozygosity for a mutation involving insertion of adenine at nucleotide 191 (exon 3); this change had created a premature termination at codon 63 (Fig. 1a) and a consequently truncated protein. We identified case 2 as compound heterozygosity for the common V279F mutation and a novel I317N mutation. The latter had substituted adenine for thymine T→A at nucleotide 950 in exon 10, resulting in substitution of Asn for Ile at codon 317 (I317N) (Fig. 1b). This mutation would create a new N-linked glycosylation site (N-X-S) on the Lp-PLA2 protein. Both mutations were confirmed by PCR-RFLP assays using restriction enzyme Tru9I (data not shown).
Nucleotide sequences of two novel mutations, InsA191 and I317N, of the Lp-PLA2 (PAF-AH) gene in two Japanese patients. a Case 1: insertion of nucleotide A at codon 63 in exon 3, which created a premature termination codon (TAA). b Case 2: T-to-A substitution at codon 317 in exon 10, which replaced a codon for isoleucine (ATC) codon with aspartate (AAC)
Impaired secretion of Lp-PLA2 from macrophages carrying InsA191 and I317N mutant alleles
We examined secretion of Lp-PLA2 protein into culture medium from stimulated endogenous monocyte-derived macrophages obtained from each of the two patients. Macrophages of case 1 secreted Lp-PLA2 protein into the medium at half the level of wild type (42.6 ng/mg cell protein versus 82.8 ng/mg cell protein), demonstrating that the truncating mutation, InsA191, indeed caused partial Lp-PLA2 deficiency in that patient (Fig. 2a). No detectable Lp-PLA2 protein was secreted by stimulated macrophages from the compound heterozygote (case 2). However, RT-PCR experiments showed that Lp-PLA2 mRNA was expressed to same extent in those macrophages (Fig. 2b).
Secretion of Lp-PLA2 by cultured monocyte-derived macrophages. a Measurement of Lp-PLA2 mass by ELISA in the culture medium on day 7. Values represent the mean ± SD of triplicate assays from two separate experiments. Wild wild type, HE heterozygote, HO homozygote. Case 1, InsA191 heterozygote; Case 2, compound heterozygote for V279F and I317N mutations. b RT-PCR showing expression of Lp-PLA2 mRNA in macrophages. M size marker, lane 1 wild type, lane 2 V279F heterozygote, lane 3 V279F homozygote, lane 4 case 1, lane 5 case 2
Expression of 317N Lp-PLA2 mutant in COS-7 cells
To clarify the molecular mechanism underlying the I317N missense variation, mutant 317N cDNA was transfected into COS-7 cells and expressed in parallel with expression vectors for wild type or 279F and 281R mutant alleles. Values for Lp-PLA2 mass and enzymatic activity, indicated in Fig. 3 by clear or black bars, respectively, show that the previously known common mutant 279F produced neither Lp-PLA2 mass nor activity in COS-7 cells and that the rare 281R allele produced one tenth the mass and activity of the wild-type protein. The novel I317N mutant from case 2 failed to produce any Lp-PLA2 mass or enzymatic activity in transfected cells. Western blotting verified the lack of mutant Lp-PLA2 proteins in lysates of COS-7 cells transfected with the mutant vectors corresponding to I317N or V279F (data not shown).
Lp-PLA2 mass and enzymatic activity in media after COS-7 cultures were transfected separately with expression constructs of different mutant alleles. Lp-PLA2 mass (clear bars) and enzymatic activity (black bars) were measured 48 h after transfection. Values represent the mean ± SD of triplicate assays from two separate experiments
Discussion
In the present study, we identified and functionally characterized two novel mutations of the Lp-PLA2 gene, InsA191 and I317N, in Japanese subjects with deficiencies in plasma Lp-PLA2 levels. The InsA191 mutation, which created a premature termination, was present on one allele of the gene in a diabetic (NIDDM) patient with partial Lp-PLA2 deficiency. Monocyte-derived macrophages from this subject secreted only half the wild-type amount of Lp-PLA2 enzyme in vitro.
The I317N mutation, which would create a new N-linked glycosylation site (N317-N-S) in the enzyme’s catalytic domain, was found in a subject with hyperlipidemia and coronary heart disease whose plasma showed complete Lp-PLA2 deficiency due to compound heterozygosity for 317N and 279F. The wild-type amino acid sequence of human Lp-PLA2 (Tjoelker et al. 1995) contains two conserved N-linked glycosylation sites, N423-T-T and N433-S-S, close to the carboxy terminus. Among members of the PLA2 family, glycosylation reduces association of the enzymes with lipoproteins but does not reduce secretion or enzymatic activity (Tselepis et al. 2001). In our experiments, neither monocyte-derived macrophages nor COS-7 cells transfected with a mutant (317N) cDNA expression vector secreted any Lp-PLA2, suggesting that this mutation disturbs processes leading to secretion of mature enzyme. These results verified that both of the novel Lp-PLA2 mutations described here indeed impair proper function of the enzyme.
Lp-PLA2 is also known as PAF-AH, since one of its known functions is to degrade and deactivate PAF and other bioactive phospholipids that mediate inflammatory disease (Stafforini et al. 1997). In some studies, a deficiency of plasma Lp-PLA2 was three times more common in children with severe bronchial asthma than in the general population, suggesting that Lp-PLA2 may play an important role in inflammatory and allergic responses (Stafforini et al. 1999). Several other investigations have revealed that Lp-PLA2 mutations increase susceptibility to inflammatory and allergic diseases (Watson et al. 1995; Tjoelker and Stafforini 2000). The two patients examined in this study did not have histories of allergic diseases, but the data suggest that individuals with deficiencies of plasma Lp-PLA2 are at increased risk of severe responses to specific events that cause allergic or inflammatory overload.
The Lp-PLA2 enzyme is thought to have a protective role in atherosclerosis because numerous reports have associated Lp-PLA2 deficiency with various cardiovascular diseases and because this enzyme catabolizes atherogenic oxidized or fragmented lipids. In fact, deficiency of Lp-PLA2 has been shown to be a predisposing factor for cardiovascular diseases in Japan. On the other hand, recent epidemiological studies carried out in Caucasian populations have suggested that the enzyme might be a positive risk factor for cardiovascular diseases. For example, Packard, Caslake and their colleagues have described a positive association between plasma Lp-PLA2 levels and incidence of coronary heart disease in case-controlled studies among males in Scotland (Caslake et al. 2000; Packard et al. 2000). A similar association between plasma Lp-PLA2 mass and coronary heart disease has been observed in Caucasian females (Blake et al. 2001).
The apparent discrepancy between suggestions of a negative role for Lp-PLA2 in atherosclerosis versus a protective role based on data involving Japanese patients with Lp-PLA2 deficiency appears to reflect the complex nature of the metabolism and activity of this enzyme as well as ethnic specificity of the genetic variations present in Japanese and Caucasian populations. These points need to be carefully examined before a full understanding of the role of this enzyme in vascular and other human diseases can be achieved.
References
Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. (2001) A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 38:1302-1306
Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. (2000) Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 150:413-419
Caslake MJ, Packard CJ (2003) Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol 14:347–352
Friedewald WT, Levy FI, Fredickson DS (1972) Estimation of the concentration of the low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–509
Fujita Y, Ezura Y, Emi M, Ono S, Takada D, Takahashi K, Uemura K, Iino Y, Katayama Y, Bujo H, Saito Y (2003) Hypertriglyceridemia associated with amino acid variation N985Y of RP1 gene. J Hum Genet 48:305–308
Haga H, Yamada R, Ohnishi Y, Nakamura Y, Tanaka T (2002) Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190,562 genetic variations in the human genome. J Hum Genet 47:605–610
Hattori H, Hirayama T, Nobe Y, Nagano M, Kujiraoka T, Egashira T, Ishii J, Tsuji M, Emi M (2002) Eight novel mutations and functional impairments of the LDL receptor in familial hypercholesterolemia in the north of Japan. J Hum Genet 47:80–87
Hiramoto M, Yoshida H, Inaizumi T, Yoshimizu N, Sato K (1997) A mutation in plasma platelet-activating factor acetylhydrolase (Val279→Phe) is a genetic risk factor for stroke. Stroke 28:2417–2420
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2002) Catalog of 77 single-nucleotide polymorphisms (SNPs) in the carbohydrate sulfotransferase 1 (CHST1) and carbohydrate sulfotransferase 3 (CHST3) genes. J Hum Genet 47:14–19
Ishii J, Nagano M, Kujiraoka T, Ishihara M, Egashira T, Takada D, Tsuji M, Hattori H, Emi M (2002) Clinical variant of Tangier disease in Japan: mutation of the ABCA1 gene in hypoalphalipoproteinemia with corneal lipidosis. J Hum Genet 47:366–369
Kosaka S, Takahashi S, Masamura K, Kanehara H, Sakai J, Tohda G, Okada E, Oida K, Iwasaki T, Hattori H, Kodama T, Yamamoto T, Miyamori I (2001) Evidence of macrophage foam cell formation by very low-density lipoprotein receptor: Interferon-g inhibition of very low-density lipoprotein receptor expression and foam cell formation in macrophages. Circulation 103:1142–1147
Kujiraoka T, Iwasaki T, Ishihara M, Ito M, Nagano M, Kawaguchi A, Takahashi S, Ishii J, Tsuji M, Egashira T, Stepanova IP, Miller NE, Hattori H (2003) Altered distribution of plasma PAF-AH between HDLs and other lipoproteins in hyperlipidemia and diabetes mellitus. J Lipid Res 44:2006–2014
Miwa M, Miyake T, Yamanaka T, Sugatani J, Suzuki Y, Sakata S, Araki Y, Matsumoto M (1988) Characterization of serum platelet-activating factor (PAF) acetylhydrolase. Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest 82:1983–1991
Nagano M, Yamashita S, Hirano K, Ito M, Maruyama T, Ishihara M, Sagehashi Y, Oka T, Kujiraoka T, Hattori H, Nakajima N, Egashira T, Kondo M, Sakai N, Matsuzawa Y (2002) Two novel missense mutations in the CETP gene in Japanese hyperalphalipoproteinemic subjects: high-throughput assay by Invader assay. J Lipid Res 43:1011–1018
Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Suzuki H, Nakamura Y (2001) A high-throughput SNP typing system for genome-wide association studies. J Hum Genet 46:471–477
Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. (2000) Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med 343:1148-1155
Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2002) Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR). J Hum Genet 47:147–171
Snyder F (1995) Platelet-activating factor and its analogs: metabolic pathways and related intracellular processes. Biochim Biophys Acta 1254:231–249
Stafforini DM, Prescott SM, McIntyre TM (1987a) Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem 262:4223–4230
Stafforini DM, McIntyre TM, Carter ME, Prescott SM (1987b) Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem 262:4215–4222
Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM (1992) The platelet-activating factor acetylhydrolase from human plasma prevents oxidative modification of low-density lipoprotein. Trans Assoc Am Physicians 105:44–63
Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM (1996a) Mammalian platelet-activating factor acetylhydrolases. Biochim Biophys Acta 1301:161–173
Stafforini DM, Satoh K, Atkinson DL, Tjoelker LW, Eberhardt C, Yoshida H, Imaizumi T, Takamatsu S, Zimmerman GA, McIntyre TM, Gray PW, Prescott SM (1996b) Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest 97:2784–2791
Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM (1997) Platelet-activating factor acetylhydrolases. J Biol Chem 272:17895–17898
Stafforini DM, Numao T, Tsodikov A, Vaitkus D, Fukuda T, Watanabe N, Fueki N, McIntyre TM, Zimmerman GA, Makino S, Prescott SM (1999) Deficiency of platelet-activating factor acetylhydrolase is a severity factor for asthma. J Clin Invest 103:989–997
Steinbrecher UP, Pritchard PH (1989) Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res 30:305–315
Stremler KE, Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM (1989) An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J Biol Chem 264:5331–5334
Stremler KE, Stafforini DM, Prescott SM, McIntyre TM (1991) Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem 266:11095–11103
Takada D, Emi M, Ezura Y, Nobe Y, Kawamura K, Iino Y, Katayama Y, Xin Y, Wu LL, Larriga-Shum S, Stephenson SH, Hunt SC, Hopkins PN (2002) Interaction between the LDL-receptor gene bearing a novel mutation and a variant in the apolipoprotein A-II promoter: molecular study in a 1135-member familial hypercholesterolemia kindred. J Hum Genet 47:656–664
Tjoelker LW, Stafforini DM (2000) Platelet-activating factor acetylhydrolases in health and disease. Biochim Biophys Acta 1488:102–123
Tjoelker LW, Wilder C, Eberhardt C, Staforini DM, Dietsch G, Schimpf B, Hooper S, Le Trong H, Cousens LS, Zimmerman GA, Yamada Y, McIntyre TM, Prescott SM, Gray PW (1995) Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 374:549–553
Tselepis AD, Karabina SP, Stengel D, Piedagnel R, Chapman MJ, Nino E (2001) N-linked glycosylation of macrophage-derived PAF-AH is a major determinant of enzyme association with plasma LDL. J Lipid Res 42:1645–1654
Unno N, Nakamura T, Kaneko H, Uchiyama T, Yamamoto N, Sugatani J, Miwa M, Nakamura S (2000) Plasma platelet-activating factor acetylhydrolase deficiency is associated with atherosclerotic occlusive disease in Japan. J Vasc Surg 32:263–267
Unno N, Nakamura T, Mitsuoka H, Uchiyama T, Yamamoto N, Saito T, Sugatani J, Miwa M, Nakamura S (2002) Association of a G994→T missense mutation in the plasma platelet-activating factor acetylhydrolase gene with risk of abdominal aortic aneurysm in Japanese. Ann Surg 235:297–302
Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, McIntyre TM, Du BN, Fogelman AM, Berliner JA (1995) Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 95:774–782
Yamada Y, Ichihara S, Fujimura T, Yokota M (1998) Identification of the G994-T missense in exon 9 of the plasma platelet-activating factor acetylhydrolase gene as an independent risk factor for coronary artery disease in Japanese men. Metabolism 47:778–781
Yamada Y, Yoshida H, Ichihara S, Imaizumi T, Satoh K, Yokota M (2000) Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Atherosclerosis 150:209–216
Yoshida S, Harada H, Nagai H, Fukino K, Teramoto A, Emi M (2002) Head-to-head juxtaposition of Fas-associated phosphatase-1 (FAP-1) and c-Jun NH2-terminal kinase 3 (JNK3) genes: genomic structure and seven polymorphisms of the FAP-1 gene. J Hum Genet 47:614–619
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishihara, M., Iwasaki, T., Nagano, M. et al. Functional impairment of two novel mutations detected in lipoprotein-associated phospholipase A2 (Lp-PLA2) deficiency patients. J Hum Genet 49, 302–307 (2004). https://doi.org/10.1007/s10038-004-0151-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-004-0151-6
Keywords
This article is cited by
-
A previously unreported impact of a PLA2G7 gene polymorphism on the plasma levels of lipoprotein-associated phospholipase A2 activity and mass
Scientific Reports (2016)
-
Effects of A379V variant of the Lp-PLA 2 gene on Lp-PLA2 activity and markers of oxidative stress and endothelial function in Koreans
Journal of Thrombosis and Thrombolysis (2014)
-
Lp-PLA2 as a Marker of Cardiovascular Diseases
Current Atherosclerosis Reports (2010)
-
Biology of Platelet-activating Factor Acetylhydrolase (PAF-AH, Lipoprotein Associated Phospholipase A2)
Cardiovascular Drugs and Therapy (2009)