Abstract
A couple was referred for exploration of repetitive abortions. The man was found to be a carrier of a balanced reciprocal translocation t(9;10)(q11;p11.1). The meiotic segregation of chromosomes 9 and 10 was analysed in 5,157 spermatozoa from this translocation carrier and in 15,255 spermatozoa from three control donors using three-colour fluorescence in situ hybridisation (FISH). The theoretical viability of the different segregation patterns was performed using the computer system HC Forum developed by the Department of Cytogenetics at the Grenoble University Medical School, La Tronche, France. A normal or balanced constitution was found in 56.25% of the analysed spermatozoa. The tertiary 3:1 segregation mode was the most frequently observed (14.37%). The frequencies of adjacent-1, adjacent-2 and 3:1 interchange modes were 12.85, 9.38 and 7.14% respectively. The cumulative frequency of non-viable imbalance was estimated at 20.91% according to the theorical viability of the different segregation patterns. Spermatozoa aneuploidy frequency was also evaluated for chromosomes X, Y and 18, and there was no evidence of interchromosomal effect in spermatozoa from the translocation carrier. FISH analysis of spermatozoa in combination with the viability theorical estimation of the different segregation patterns could be considered a useful tool for genetic counselling in carriers of reciprocal translocation.
Similar content being viewed by others
Introduction
Reciprocal translocations are one of the most frequent structural rearrangements in man (approximately 1.4 in 1,000 newborns) (Nielsen and Wohlert 1991). Equilibrated reciprocal translocations are in most of cases silent but they expose their carriers to reproductive failure (infertility, spontaneous abortion and death-birth children) or chromosomally unbalanced offspring. These abnormalities result from an unequilibrated meiotic segregation mode (adjacent-1, adjacent-2, 3:1 tertiary or 3:1 interchange) while normal and balanced conceptus are generated by alternate segregation (Cans et al. 1993). The prediction of unbalanced mode depends on several factors: (1) meiotic factors (centromere and chiasma position), (2) selective factors (ascertainment of the translocation), (3) size of the translocated segments and (4) presence or absence of heterochromatin. Translocations ascertained by the occurrence of repeated abortions suggest that these imbalances are unviable and the risk of abnormal progeny at birth is considered low. Other translocations can be diagnosed by viable unbalanced offspring, and the risk of deficiency for the progeny is high. Finally, some translocations could be ascertained during exploration of a male-factor infertility associated to sperm parameter alterations. However, in this instance, potential viability of the progeny remains unknown.
Different theorical methods have been developed for predicting the risk of imbalances at birth and the preferential mode of segregation for each individual translocation: for example, the “pachytene diagram predictive method” (PDP method) based on the analysis of rough lengths of centric and translocated segments (Jalbert and Sèle 1980) and the “discriminant method” (D method) taking into account specific chromosomal information concerning the translocation or characteristics of the carrier parent (Cans et al. 1993). However, karyotypes or fluorescence in situ hybridisation (FISH) analysis of sperm products from reciprocal translocation male carriers is a complement to epidemiological studies to evaluate the distribution of the different meiotic segregation patterns and consequently the proportion of normal, balanced and unbalanced spermatozoa.
This study provides FISH results on the spermatozoa segregation patterns from a male carrier of a reciprocal translocation t(9;10)(q11;p11.1). The risk of unbalanced, viable progeny as well as the theorical risk of spontaneous abortion are evaluated, taking into account the percentage of viable and non-viable conceptus.
Materials and methods
Probands
The patient studied was a 25-year-old man who was referred to our centre for infertility exploration. The couple previously had four first-trimester spontaneous abortions. The patient had no clinical history, and the physical examination was normal. Semen analysis revealed moderate sperm alterations with a primary asthenospermia and a teratospermia (sperm count 57×106/ml; motility (a+b) 25%; normal morphology 33%). His constitutional karyotype established with RHG banding and prometaphase chromosome preparation revealed a (9;10)(q11;p11.1) balanced reciprocal translocation.
Three healthy probands of proven fertility, aged between 28 and 30 years, with normal semen parameters and a normal chromosome constitution, were included in the study as a control group.
Sperm preparation
Sperm samples were fixed in methanol:glacial acetic acid for 60 min at 4°C and smeared onto clean glass slides (SuperFrost/Plus, Menzel-Gläser, Germany). Sperm nuclei decondensation was performed for 1–2 min in 25 mM DTT, 1 M TrisHCl pH9.5.
DNA probes
Five different probes were used in the study:
-
Three probes were required for the translocation analysis: (1) Alphasatellite centromeric probes specific for chromosomes 9 and 10 labelled with spectrum orange (CEP 9) and spectrum green (CEP 9; CEP 10) purchased from Adgenix (Voisins Le Bretonneux, France); (2) A YAC clone (908F11), specific for chromosome 9q22, insert size 1290 kb (CEPH, Fondation Jean Dausset, France), labelled by nick translation with biotin-dATP (Gibco BRL, France). The efficiency of the labelling was controlled on metaphase and interphase nuclei obtained from peripheral blood cells.
-
Probes for sex chromosomes and chromosome 18 were used to evaluate an interchromosomal effect generated by the reciprocal translocation. These probes were directly labelled by the manufacturer (Adgenix) with spectrum orange (CEP 18; CEP Y) or spectrum green (CEP Y; CEP X).
Fluorescence in situ hybridisation in sperm nuclei
Two FISH assays were performed: (1) a three-colour FISH using centromeric probes for chromosomes 9 (yellow colour) and 10 (green colour) and the YAC probe 9q22 (red colour); (2) a three-colour FISH with the probes for chromosomes X (green), Y (yellow) and 18 (red). The yellow colour was obtained by mixing an equal volume of the same probe labelled with spectrum orange and spectrum green (Adgenix).
After denaturation of probes and slides, hybridisation was carried out at 37°C overnight in a moist chamber. Detection of the labelling sites of the YAC probe was performed by incubations with avidin Texas-red/biotinylated anti-avidin (Cambio, Adgenix).
Scoring criteria and data collection
FISH analysis was performed with a ×100 objective in an epifluorescence microscope (DMRD, Leica, Germany) equipped with a triple-band pass filter (FITC/Rhodamine/DAPI). Images were captured and produced at ×1,000 magnification with a digital imaging system (Mac Probe version 3.3; Perceptive Scientific International LTD, Chester, England).
Slides were included for scoring if the hybridisation efficiency was >95%. At least 10,000 sperm nuclei were scored for the segregation analysis of the translocation and the aneuploidy assay of chromosomes X, Y and 18. Some spermatozoa were excluded from the count: diffuse fluorescent domains, excessive decondensation and overlapping nuclei. Fluorescent spots were retained if they had comparable size and intensity and they were separated by at least one spot diameter.
For three-colour FISH with chromosomes X, Y and 18, two fluorescent spots of comparable size and intensity separated by at least one diameter of the domain of one signal were considered as two copies of the corresponding chromosome. The predicted segregation patterns of chromosomes 9 and 10, as well as the predicted fluorescent signals of the sperm nuclei after alternate, adjacent-1, adjacent-2 and 3:1 segregation at meiosis 1, are represented in Fig. 1. Data were statistically analysed with the logicial Statview (Abacus Concepts, Inc., Berkeley, CA, USA) using a chi-square test. A p value <0.05 was considered to be significant.
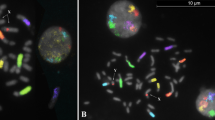
RTG-banded chromosomes from the t(9;10)(q11;p11.1) carrier showing the normal and the derivative abnormal chromosomes 9 and 10 (A). Pachytene quadrivalent shows chromosome pairing between chromosome 9 and 10. Fluorescence in situ hybridisation on decondensed spermatozoa of a t(9;10) carrier using centromeric probe for chromosomes 9 (yellow) and 10 (green) and a biotinylated YAC clone (908F11), specific for chromosome 9q22 (red) corresponded to six different signal patterns: Normal or balanced spermatozoon (B), unbalanced spermatozoa (C, D, E, F, G)
Results
Triple FISH on lymphocytes
Probes were hybridised on human lymphocyte nuclei and metaphases to determine the hybridisation efficiency. Triple FISH was performed on 64 metaphases and 125 interphase nuclei. Overall, 98.4% of the cells displayed the expected normal diploid constitution, while the remaining cells showed loss of signals due to hypoploidy or hybridisation failure.
Segregation patterns of reciprocal translocations
t(9;10)(q11;p11.1) carrier
A total of 5,157 spermatozoa were analysed to determine the meiotic segregation of the reciprocal translocation (Fig. 1). The numbers and percentages of each type of segregation observed are reported in Table 1. Most segregation products resulted from 2:2 segregation (78.28%) or 3:1 segregation (21.72%). The 4:0 segregation was not observed. The segregation alternate resulting in balanced spermatozoa (normal karyotype or balanced translocation) was the most frequently observed (56.25%). Among the unbalanced segregation products, the 3:1 tertiary segregation accounted for 14.37%. The adjacent-1 and adjacent-2 segregations (12.85% and 9.38% respectively), as well as the 3:1 interchange segregation (7.14%), were less frequently observed than the other segregation products. Overall, 43.74% spermatozoa carried an unbalanced segregation product, 21.51% of them had 22 or 24 chromosomes.
Control group
A total of 15,255 spermatozoa were included in the count with a rate of disomy frequency for chromosomes 9 and 10 respectively estimated to 0.28±0.05% and 0.27±0.02%. The rate of diploid sperm nuclei was estimated to 0.05±0.02%.
Aneuploidy frequency for chromosomes not involved in the translocation
Triple FISH using probes for chromosomes X, Y and 18 was performed in three colour FISH on 10,097 spermatozoa from the translocation carrier and on 30,202 sperm nuclei from the control group (Table 1). The ratio of X- to Y-bearing spermatozoa did not significantly differ from 1:1. The incidence of XX, YY and XY sperm nuclei was not significantly increased when compared to controls. The same data were observed for the frequency of chromosome 18 disomy, as well as for the rate of diploidy. An interchromosomal effect was excluded for the chromosomes X, Y and 18.
Discussion
The result of translocation segregation mode patterns could be evaluated, in males, by sperm karyotypes or by FISH on spermatozoa. FISH analysis explores larger number of spermatozoa than karyotypes performed by human-hamster system. Sperm karyotypes are able to evaluate separately the result of the different segregation modes. FISH studies in dual colour, using in most cases centromeric probes for the chromosomes involved in the translocation, do not allow to differentiate alternate and adjacent-1 segregants (Spriggs and Martin 1994; Rousseaux et al. 1995; Mennicke et al. 1997; Estop et al. 1997; Mercier et al. 1998; Giltay et al. 1999). One exception was the translocation (7;8)(q11;q21;cen) reported by Mercier et al. (1998). Dual-colour FISH with centromeric probes enabled the authors to differentiate between alternate and adjacent-1 segregation because one of the breakpoints had a centromeric position.
In our study, as in other published data (Cifuentes et al. 1999; Van Hummelen et al. 1997; Estop et al. 1998; Blanco et al. 1998 Martini et al. 1998; Geneix et al. 2002), a three-colour FISH analysis using a locus-specific probe for the translocated fragment and centromeric probes has enabled the detection of all types of segregations. The differentiation of adjacent-1 and alternate segregation was also possible because the interstitial segment at meiosis 1 is short, reducing considerably the probability of an interstitial chiasma (Armstrong and Hulten 1998). However, the combination of these three probes did not allow to differentiate the products of an alternate segregation (normal or balanced) or between 4:0 segregations and diploid spermatozoa.
The translocation explored in our study has never, to our knowledge, been reported in the literature or in other databases, such as HC Forum (https://hcforum.imag.fr, Dr. O. Cohen, Grenoble, France). The frequency of unbalanced spermatozoa was estimated at 43.74%, with 22.33% of sperm nuclei with partial monosomy and trisomy and 21.51% of them with complete monosomy and trisomy. This frequency was not significantly different from the rate of balanced spermatozoa (56.25%). The frequency of unbalanced gametes varies from one translocation to another (Shi and Martin 2001), as does the risk for reciprocal translocation carriers of producing unbalanced offspring. The risk of imbalance at birth also depends on the probabilities for the survival of unbalanced zygotes, embryos, foetuses or newborn. Both these two probabilities depend on the chromosomes involved in the translocation and on break-point location (Midro et al. 1992).
Unbalanced spermatozoa resulting from the 3:1 segregation mode were predominant in our patient, with a global frequency of 21.51% (14.37% from the 3:1 tertiary mode and 7.14% from the 3:1 interchange mode). However, data arising from sperm karyotypes and FISH suggest that (i) adjacent-1 mode is the most frequent imbalance observed, and (2) adjacent-2 mode is more prevalent than 3:1 mode (Shi and Martin 2001). This was not observed in our patient, where the frequency of adjacent-2 segregation occurred at a frequency of only 9.38%. The same data were reported in four published reciprocal translocations t(3;11)(q27.3;q24.3) (Martini et al. 1998), t(11;22)(q23;q11) (Estop et al. 1997), t(11;22)(q25;q12) (Van Assche et al. 1999) and t(17;22)(q11;q12) (Geneix et al. 2002).
Analysis of chromosomally unbalanced offspring has shown that: (1) translocations producing adjacent-2 and 3:1 imbalances frequently involve acrocentric chromosomes or chromosome 9 and (2) chromosome 9 is less associated to adjacent-1 imbalances transmitted by men than by women (Faraut et al. 2000). Acrocentric chromosome was implicated in translocations described by Estop et al. (1998), Van Assche et al. (1999) and Geneix et al. (2002), while chromosome 9 interested our reciprocal translocation. After a 3:1 mode of segregation, a gamete with 24 chromosomes and a complementary gamete with 22 chromosomes should normally be produced. This was observed, in our case for the 3:1 tertiary mode but not for the 3:1 interchange mode. A technical artefact as regards the hybridisation efficiency of the smallest probe may explain the higher incidence of nullisomia compared to disomia, as suggested by Martini et al. (1998) and Geneix et al. (2002). In the second scenario, gametes with 24 chromosomes were observed at a lower frequency than those with 22 chromosomes (ratio 1:10). The same ratio was reported by Van Assche et al. (1999). Estop et al. (1998) observed the same data and proposed that spermatocytes with 22 chromosomes have an increased ability to survive a 3:1 segregation. This point appears to be in opposition with the lethality for conceptus of autosomal monosomia. The segregation mode patterns of our translocation are in agreement with the predictive estimation of imbalance at birth evaluated by the data base HC Forum (Cans et al. 1993). This data base has indicated that the most probable imbalance at birth for the (9;10) translocation was the 3:1 tertiary mode with a frequency of unbalanced live birth of 3.5%.
However, taking into account that each potential imbalance could be defined from the percentages of monosomy and trisomy related to the total haploid genome (Cohen et al. 1994), the frequency of viable unbalanced segregants could be modified (Table 1). In this situation, for our translocation carrier, the mode adjacent-1 presents the highest frequency (12.85%) of producing viable unbalanced live birth, then the mode adjacent-2 (9.38%) and finally the mode 3:1 (0.6%). The frequency of lethal unbalanced segregants could be estimated at 20.91% and corresponds to the theorical risk of miscarriages estimated in our translocation carrier. The combination of FISH results and theorical risk offers more specific information for genetic counselling. Furthermore, our translocation carrier was ascertained by recurrent miscarriages and was a candidate for an IVF procedure. The estimation of imbalance at birth as well as the risk of miscarriage are data of major interest for an effective management of couples.
The presence of an increased frequency of chromosome abnormalities unrelated to the translocation remains controversial. The disomy frequency for chromosomes X, Y and 18 was not increased in our patient compared to controls. We observed no increased diploidy frequency in the male carrier. The presence of an interchromosomal effect varied among the chromosomes explored and the different studies have not analysed the same chromosome (Blanco et al. 2000; Estop et al. 2000; Vegetti et al. 2000; Pellestor et al. 2001). The sex chromosomes as well as chromosome 21 were the most frequently evaluated. Sex chromosome disomy frequency was not significantly different from controls in most of carriers. Chromosome 21 was also the most frequently affected by non-disjunctions in translocations carriers (12 of 38 probands).
The occurrence of an interchromosomal effect could depend on the quality of semen parameters The risk for interchromosomal effect appears to be real in the infertile male carrier of translocations (Vegetti et al., 2000; Pellestor et al., 2001). They observed that infertile patients with chromosomal rearrangement showed a higher incidence of diploidy and disomy compared to controls. This was not detected in our patient who presented an asthenoteratospermia. These results raised the question of the origin of these chromosome abnormalities: (1) Are they related to the structural rearrangement and due to an interchromosomal effect or (2) in relation to a deleterious environment responsible of sperm alterations and meiotic abnormalities? These hypothesis need to be confirmed by FISH analysis of a larger number of reciprocal translocation carriers ascertained by the exploration of sperm alterations. The majority of the published translocations are discovered by repetitive miscarriages and, in most cases, no information is reported regarding the result of semenogram.
References
Armstrong SJ and Hulten MA (1998) Meiotic segregation analysis by FISH investigations in sperm and spermatocytes of translocation heterozygotes. Eur J Hum Genet 6:430–431
Blanco J, Egozcue J, Clusellas N, Vidal F (1998) FISH on sperm heads allows the analysis of chromosome segregation and interchromosomal effects in carriers of structural rearrangements: results in a translocation carrier, t(5;8)(q33;q13). Cytogenet Cell Genet 83:275–280
Blanco J, Egozcue J, Vidal F (2000) Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet 106:500–505
Cans C, Cohen O, Lavergne C, Mermet MA, Demongeot J, Jalbert P (1993) Logistic regression model to estimate the risk of unbalanced offspring in reciprocal translocations. Hum Genet 92:598–604
Cans C, Cohen O, Mermet MA, Demongeot J, Jalbert P (1993) Human reciprocal translocations: is the unbalanced mode at birth predictable? Hum Genet 91:228–232
Cifuentes P, Navarro J, Blanco J, Vidal F, Miguez L, Egozcue J, Benet J (1999) Cytogenetic analysis of sperm chromosomes and sperm nuclei in a male heterozygous for a reciprocal translocation t(5;7)(q21;q32) by in situ hybridisation. Eur J Hum Genet 7:231–238
Cohen O, Cans C, Mermet MA, Demongeot J, Jalbert P (1994) Viability thresholds for partoal trisomies and monosomies. A study of 1,159 viable unbalanced reciprocal translocations. Hum Genet 93:188–194
Cohen O, Cans C, Cuillel M, Gilardi JL, Roth H, Mermet MA, Jalbert P, Demongeot J (1996) Cartographic study: breakpoints in 1574 families carrying human reciprocal translocations. Hum Genet 97:659–667
Estop AM, Cieply KM, Aston CE (1997) The meiotic segregation pattern of a reciprocal translocation t(10;12)(q26.1;p13.3) by fluorescence in situ hybridization sperm analysis. Eur J Hum Genet 5:78–82
Estop AM, Cieply KM, Wakim A, Feingold E (1998) Meiotic products of two reciprocal translocations studied by multicolor fluorescence in situ hybridization. Cytogenet Cell Genet 83:193–198
Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E (2000) Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet 106:517–524
Faraut T, Mermet MA, Demongeot J, Cohen O (2000) Cooperation of selection and meiotic mechanisms in the production of imbalances in reciprocal translocations. Cytogenet Cell Genet 88:15–21
Geneix A, Schubert B, Force A, Rodet K, Briancon G, Boucher D (2002) Sperm analysis by FISH in a case of t(17; 22) (q11; q12) balanced translocation: case report. Hum Reprod 17:325–331
Giltay JC, Kastrop PM, Tiemessen CH, van Inzen WG, Scheres JM, Pearson PL (1999) Sperm analysis in a subfertile male with a Y;16 translocation, using four-color FISH. Cytogenet Cell Genet 84:67–72
Jalbert P, Sele B, Jalbert H (1980) Reciprocal translocations: a way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum Genet 55:209–222
Martini E, von Bergh AR, Coonen E, de Die-Smulders CE, Hopman AH, Ramaekers FC, Geraedts JP (1998) Detection of structural abnormalities in spermatozoa of a translocation carrier t(3;11)(q27.3;q24.3) by triple FISH. Hum Genet 102:157–165
Mennicke K, Diercks P, Schlieker H, Bals-Pratsch M, al Hasani S, Diedrich K, Schwinger E (1997) Molecular cytogenetic diagnostics in sperm. Int J Androl 20 [Suppl 3] 11–19
Mercier S, Morel F, Fellman F, Roux C, Bresson JL (1998) Molecular analysis of the chromosomal equipment in spermatozoa of a 46, XY, t(7;8) (q11.21;cen) carrier by using fluorescence in situ hybridization. Hum Genet 102:446–451
Midro AT, Stengel-Rutkowski S, Stene J (1992) Experiences with risk estimates for carriers of chromosomal reciprocal translocations. Clin Genet 41:113–122
Nielsen J, Wohlert M (1991) Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet 87:81–83
Pellestor F, Imbert I, Andreo B, Lefort G (2001) Study of the occurrence of interchromosomal effect in spermatozoa of chromosomal rearrangement carriers by fluorescence in-situ hybridization and primed in-situ labelling techniques. Hum Reprod 16:1155–1164
Rousseaux S, Chevret E, Monteil M, Cozzi J, Pelletier R, Devillard F, Lespinasse J, Sele B (1995) Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet 71:240–246
Shi Q, Martin RH (2001) Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction 121:655–666
Spriggs EL, Martin RH (1994) Analysis of segregation in a human male reciprocal translocation carrier, t(1;11) (p36.3;q13.1), by two-colour fluorescence in situ hybridization. Mol Reprod Dev 38:247–250
Van Assche E, Staessen C, Vegetti W, Bonduelle M, Vandervorst M, Van Steirteghem A, Liebaers I (1999) Preimplantation genetic diagnosis and sperm analysis by fluorescence in- situ hybridization for the most common reciprocal translocation t(11;22). Mol Hum Reprod 5:682–690
Van Hummelen P, Manchester D, Lowe X, Wyrobek AJ (1997) Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet 61:651–659
Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Van Steirteghem A (2000) Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod 15:351–365
Acknowledgements
The authors thank Mr. R. Medeiros for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The experiments performed in this study comply with the current French laws.
Rights and permissions
About this article
Cite this article
Rives, N., Jarnot, M., Mousset-Siméon, N. et al. Fluorescence in situ hybridisation (FISH) analysis of chromosome segregation and interchromosomal effect in spermatozoa of a reciprocal translocation t(9,10)(q11;p11.1) carrier. J Hum Genet 48, 535–540 (2003). https://doi.org/10.1007/s10038-003-0072-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-003-0072-9