Abstract
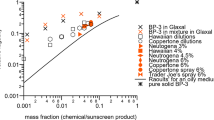
Nanoscale ingredients in commercial products represent a point of emerging environmental concern due to recent findings that correlate toxicity with small particle size. A weight-of-evidence (WOE) approach based upon multiple lines of evidence (LOE) is developed here to assess nanomaterials as they exist in consumer product formulations, providing a qualitative assessment regarding the presence of nanomaterials, along with a baseline estimate of nanoparticle concentration if nanomaterials do exist. Electron microscopy, analytical separations, and X-ray detection methods were used to identify and characterize nanomaterials in sunscreen formulations. The WOE/LOE approach as applied to four commercial sunscreen products indicated that all four contained at least 10% dispersed primary particles having at least one dimension <100 nm in size. Analytical analyses confirmed that these constituents were comprised of zinc oxide (ZnO) or titanium dioxide (TiO2). The screening approaches developed herein offer a streamlined, facile means to identify potentially hazardous nanomaterial constituents with minimal abrasive processing of the raw material.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
U.S. EPA. Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen (Final). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/057F, 2010. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=230972.
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N . Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 2012; 46: 2242–2250.
Kim T-H, Kim M, Park H-S, Shin US, Gong M-S, Kim H-W . Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A 2012; 100A: 1033–1043.
Johnston H, Hutchison G, Christensen F, Peters S, Hankin S, Stone V . Identification of the mechanisms that drive the toxicity of TiO2 particulates: the contribution of physicochemical characteristics. Part Fibre Toxicol 2009; 6: 33.
Food and Drug Administration (FDA). Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-Counter Human Use - Small Entity Compliance Guide. Silver Spring, MD, USA. 2012 http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm330694.htm.
European Commission. Commission recommendation of 18 october 2011 on the definition of nanomaterial, 2011/696/EU. Off J Eur Union 2011; L275: 38–40.
American Society for Testing and Materials (ASTM). Standard Guide for Handling Unbound Engineered Nanoscale Particles in Occupational Settings, E 2535-07. ASTM International: West Conshohocken, PA, USA. 2007.
Szakal C, Roberts S, Westerhoff P, Bartholomaeus A, Buck N, Illuminato I et al. Measurement of nanomaterials in foods: integrative consideration of challenges and future prospects. ACS Nano 2014; 8: 3128–3135.
Duncan TV . Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci 2011; 363: 1–24.
Schafer B, Tentschert J, Luch A . Nanosilver in consumer products and human health: more information required!. Environ Sci Technol 2011; 45: 7589–7590.
Lewicka ZA, Benedetto AF, Benoit DN, Yu WW, Fortner JD, Colvin VL . The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J Nanopart Res 2011; 13: 3607–3617.
Tyner KM, Wokovich AM, Doub WH, Buhse LF, Sung L-P, Watson SS et al. Comparing methods for detecting and characterizing metal oxide nanoparticles in unmodified commercial sunscreens. Nanomedicine 2009; 4: 145–159.
Noonan G, Whelton A, Carlander D, Duncan T . Measurement methods to evaluate engineered nanomaterial release from food contact materials. Compr Rev Food Sci Food Safe 2014; 13: 679–692.
Guo H, Zhang Z, Xing B, Mukherjee A, Musante C, White J et al. Analysis of silver nanoparticles in antimicrobial products using surface-enhanced raman spectroscopy (SERS). Environ Sci Technol 2015; 49: 4317–4324.
Hope BK, Clarkson JR . A strategy for using weight-of-evidence methods in ecological risk assessments. Hum Ecol Risk Assess 2013; 20: 290–315.
Hull RN, Swanson S . Sequential analysis of lines of evidence–an advanced weight-of-evidence approach for ecological risk assessment. Integr Environ Assess Manag 2006; 2: 302–311.
von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem 2012; 31: 32–49.
Poda AR, Bednar AJ, Kennedy AJ, Harmon A, Hull M, Mitrano DM et al. Characterization of silver nanoparticles using flow-field flow fractionation interfaced to inductively coupled plasma mass spectrometry. J Chromatogr A 2011; 1218: 4219–4225.
Contado C, Pagnoni A . TiO2 in commercial sunscreen lotion: flow field-flow fractionation and ICP-AES together for size analysis. Anal Chem 2008; 80: 7594–7608.
Samontha A, Shiowatana J, Siripinyanond A . Particle size characterization of titanium dioxide in sunscreen products using sedimentation field-flow fractionation-inductively coupled plasma-mass spectrometry. Anal Bioanal Chem 2011; 399: 973–978.
Acknowledgements
The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the US Government. Permission was granted by the Chief of Engineers to publish this information. The findings of this report are not to be construed as an official Department of the Army position unless so designated by other authorized documents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cuddy, M., Poda, A., Moser, R. et al. A weight-of-evidence approach to identify nanomaterials in consumer products: a case study of nanoparticles in commercial sunscreens. J Expo Sci Environ Epidemiol 26, 26–34 (2016). https://doi.org/10.1038/jes.2015.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2015.51
Keywords
This article is cited by
-
Legal and practical challenges in classifying nanomaterials according to regulatory definitions
Nature Nanotechnology (2019)
-
Influence of shear stress and size on viability of endothelial cells exposed to gold nanoparticles
Journal of Nanoparticle Research (2017)