Abstract
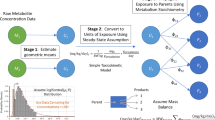
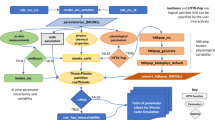
Biomarker concentrations in spot samples of blood and urine are implicitly interpreted as direct surrogates for long-term exposure magnitude in a variety of contexts including (1) epidemiological studies of potential health outcomes associated with general population chemical exposure, and (2) cross-sectional population biomonitoring studies. However, numerous factors in addition to exposure magnitude influence biomarker concentrations in spot samples, including temporal variation in spot samples because of elimination kinetics. The influence of half-life of elimination relative to exposure interval is examined here using simple first-order pharmacokinetic simulations of urinary concentrations in spot samples collected at random times relative to exposure events. Repeated exposures were modeled for each individual in the simulation with exposure amounts drawn from lognormal distributions with varying geometric standard deviations. Relative variation in predicted spot sample concentrations was greater than the variation in underlying dose distributions when the half-life of elimination was shorter than the interval between exposures, with the degree of relative variation increasing as the ratio of half-life to exposure interval decreased. Results of the modeling agreed well with data from a serial urine collection data set from the Centers for Disease Control. Data from previous studies examining intra-class correlation coefficients for a range of chemicals relying upon repeated sampling support the importance of considering the half-life relative to exposure frequency in design and interpretation of studies using spot samples for exposure classification and exposure estimation. The modeling and data sets presented here provide tools that can assist in interpretation of variability in cross-sectional biomonitoring studies and in design of studies utilizing biomonitoring data as markers for exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sexton K., Needham L.L., and Pirkle J.L. Human biomonitoring of environmental chemicals. Am Scientist 2004: 92: 38–45.
Lakind J.S., and Naiman D.Q. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examination Survey. J Exp Sci Environ Epi 2011: 21 (3): 272–279.
Calafat A.M., and McKee R.H. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ Health Persp 2006: 114 (11): 1783–1789.
Wittassek M., Koch H.M., Angerer J., and Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Molecular Nutr Food Res 2011: 55 (1): 7–31.
Clewell H.J., Tan Y.M., Campbell J.L., and Andersen M.E. Quantitative interpretation of human biomonitoring data. Toxicol Appl Pharmacol 2008: 231 (1): 122–133.
Kohn M.C., Parham F., Masten S.A., Portier C.J., Shelby M.D., and Brock J.W., et al. Human exposure estimates for phthalates. Environ Health Persp 2000: 108 (10): A440–A442.
Preau Jr J.L., Wong L.Y., Silva M.J., Needham L.L., and Calafat A.M. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Persp 2010: 118 (12): 1748–1754.
Ye X., Wong L.Y., Bishop A.M., and Calafat A.M. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Persp 2011: 119 (7): 983–988.
Teeguarden J.G., Calafat A.M., Ye X., Doerge D.R., Churchwell M.I., and Gunawan R., et al. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol Sci 2011: 123 (1): 48–57.
Li Z., Romanoff L.C., Lewin M.D., Porter E.N., Trinidad D.A., and Needham L.L., et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Exp Sci Environ Epi 2010: 20 (6): 526–535.
Ott W.R. A physical explanation of the lognormality of pollutant concentrations. J Air Waste Management Assoc 1990: 40 (10): 1378–1383.
Koch H.M., Bolt H.M., Preuss R., and Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol 2005: 79 (7): 367–376.
Volkel W., Kiranoglu M., and Fromme H. Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol Let 2008: 179 (3): 155–162.
Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services. Atlanta, GA, Available at: http://www.cdc.gov/exposurereport/[Accessed 5 January 2010] 2009.
Spaan S., Fransman W., Warren N., Cotton R., Cocker J., and Tielemans E. Variability of biomarkers in volunteer studies: the biological component. Toxicol Let 2010: 198 (2): 144–151.
Mage D.T., Allen R.H., and Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J Expo Sci Environ Epidemiol 2008: 18 (4): 360–368.
Mage D.T., Allen R.H., Gondy G., Smith W., Barr D.B., and Needham L.L. Estimating pesticide dose from urinary pesticide concentration data by creatinine correction in the Third National Health and Nutrition Examination Survey (NHANES-III). J Expo Anal Environ Epidemiol 2004: 14 (6): 457–465.
van Haarst E.P., Heldeweg E.A., Newling D.W., and Schlatmann T.J. The 24-h frequency-vlume chart in adults reporting no voiding complaints: defining reference values and analysing variables. Br J Urol Int 2004: 93: 1257–1261.
Rosner B. Fundamentals of Biostatistics. Duxbury: Pacific Grove, CA, 2000.
Adibi J.J., Whyatt R.M., Williams P.L., Calafat A.M., Camann D., and Herrick R., et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Persp 2008: 116 (4): 467–473.
Teitelbaum S.L., Britton J.A., Calafat A.M., Ye X., Silva M.J., and Reidy J.A., et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 2008: 106 (2): 257–269.
Kile M.L., Hoffman E., Hsueh Y.M., Afroz S., Quamruzzaman Q., and Rahman M., et al. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Persp 2009: 117 (3): 455–460.
Rivera-Nunez Z., Meliker J.R., Linder A.M., and Nriagu J.O. Reliability of spot urine samples in assessing arsenic exposure. Int J Hyg Environ Health 2010: 213 (4): 259–264.
Poulin P., Jones R.D.O., Jones H.M., Gibson C.R., Rowland M., and Chien J.Y., et al. PHRMA CPCDC intitiative on predictive models of human pharmacokinetics, part 5: prediction of plasma concentratin-time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J Pharmaceutical Sci 2011: 100 (10): 4127–4157.
Koch H.M., Bolt H.M., and Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004: 78 (3): 123–130.
Aylward L.L., LaKind J.S., and Hays S.M. Biomonitoring equivalents (BE) dossier for trihalomethanes. Regul Toxicol Pharmacol 2008: 51 (3 Suppl): S68–S77.
Kuijsten A., Arts I.C., Vree T.B., and Hollman P.C. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr 2005: 135 (4): 795–801.
Lorber M. Use of a simple pharmacokinetic model to characterize exposure to perchlorate. J Exp Sci Environ Epi 2009: 19 (3): 260–273.
Shelnutt S.R., Cimino C.O., Wiggins P.A., and Badger T.M. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev 2000: 9 (4): 413–419.
Metzner J.E., Frank T., Kunz I., Burger D., and Riegger C. Study on the pharmacokinetics of synthetic genistein after multiple oral intake in post-menopausal women. Arzneimittelforschung. 2009: 59 (10): 513–520.
Sandborgh-Englund G., Adolfsson-Erici M., Odham G., and Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health Part A 2006: 69 (20): 1861–1873.
Chandler H.A., Archbold G.P., Gibson J.M., O’Callaghan P., Marks J.N., and Pethybridge R.J. Excretion of a toxic dose of thallium. Clin Chem 1990: 36 (8 Part 1): 1506–1509.
Hays S.M., and Aylward L.L. Biomonitoring equivalents (BE) dossier for acrylamide (AA) (CAS No 79-06-1). Regul Toxicol Pharmacol 2008: 51 (3 Suppl): S57–S67.
Flesch-Janys D., Becher H., Gurn P., Jung D., Konietzko J., and Manz A., et al. Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. J Toxicol Environ Health 1996: 47 (4): 363–378.
Seals R., Bartell S.M., and Steenland K. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ Health Persp 2011: 119 (1): 119–124.
Brown Jr J.F., Lawton R.W., and Morgan C.B. PCB metabolism, persistence, and health effects after occupational exposure: implications for risk assessment. Chemosphere 1994: 29 (9-11): 2287–2294.
Arakawa C., Fujimaki K., Yoshinaga J., Imai H., Serizawa S., and Shiraishi H. Daily urinary excretion of bisphenol A. Environ Health Prev Med 2004: 9 (1): 22–26.
Baird D.D., Saldana T.M., Nepomnaschy P.A., Hoppin J.A., Longnecker M.P., and Weinberg C.R., et al. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J Exp Sci Environ Epi 2010: 20 (2): 169–175.
Braun J.M., Kalkbrenner A.E., Calafat A.M., Bernert J.T., Ye X., and Silva M.J., et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Persp 2011: 119 (1): 131–137.
Fromme H., Bolte G., Koch H.M., Angerer J., Boehmer S., and Drexler H., et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health 2007: 210 (1): 21–33.
Hauser R., Meeker J.D., Park S., Silva M.J., and Calafat A.M. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Persp 2004: 112 (17): 1734–1740.
Hoppin J.A., Brock J.W., Davis B.J., and Baird D.D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Persp 2002: 110 (5): 515–518.
Kissel J.C., Curl C.L., Kedan G., Lu C., Griffith W., Barr D.B., Needham L.L., and Fenske R.A. Comparison of organophosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. J Expo Anal Environ Epidemiol. 2005: 15 (2): 164–171.
Mahalingaiah S., Meeker J.D., Pearson K.R., Calafat A.M., Ye X., and Petrozza J., et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Persp 2008: 116 (2): 173–178.
Meeker J.D., Barr D.B., Ryan L., Herrick R.F., Bennett D.H., and Bravo R., et al. Temporal variability of urinary levels of nonpersistent insecticides in adult men. J Exp Anal Environ Epi 2005: 15 (3): 271–281.
Nepomnaschy P.A., Baird D.D., Weinberg C.R., Hoppin J.A., Longnecker M.P., and Wilcox A.J. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environ Res 2009: 109 (6): 734–737.
Peck J.D., Sweeney A.M., Symanski E., Gardiner J., Silva M.J., and Calafat A.M., et al. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. J Exp Sci Environ Epi 2010: 20 (1): 90–100.
Scher D.P., Alexander B.H., Adgate J.L., Eberly L.E., Mandel J.S., and Acquavella J.F., et al. Agreement of pesticide biomarkers between morning void and 24-h urine samples from farmers and their children. J Exp Sci Environ Epi 2007: 17 (4): 350–357.
Sexton K., Adgate J.L., Church T.R., Ashley D.L., Needham L.L., and Ramachandran G., et al. Children's exposure to volatile organic compounds as determined by longitudinal measurements in blood. Environ Health Persp 2005: 113 (3): 342–349.
Sexton K., and Ryan A.D. Using exposure biomarkers in children to compare between-child and within-child variance and calculate correlations among siblings for multiple environmental chemicals. J Exp Sci Environ Epi 2011.
Acknowledgements
Funding for this work was provided by the Long-Range Research Initiative of the American Chemistry Council. The authors carried out the analyses with no input from the funding organization and had complete freedom to design, execute, analyze, and report the results of their work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aylward, L., Kirman, C., Adgate, J. et al. Interpreting variability in population biomonitoring data: Role of elimination kinetics. J Expo Sci Environ Epidemiol 22, 398–408 (2012). https://doi.org/10.1038/jes.2012.35
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2012.35
Keywords
This article is cited by
-
A sensitive GC–MS/MS method for the quantification of benzo[a]pyrene tetrol in urine
Analytical and Bioanalytical Chemistry (2024)
-
Year-to-year variation in phthalate metabolites in the Midlife Women’s Health Study
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Bayesian inference of chemical exposures from NHANES urine biomonitoring data
Journal of Exposure Science & Environmental Epidemiology (2022)
-
Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry
Journal of Exposure Science & Environmental Epidemiology (2017)
-
Estimated Daily Intake and Cumulative Risk Assessment of Phthalates in the General Taiwanese after the 2011 DEHP Food Scandal
Scientific Reports (2017)