Abstract
We previously reported isolating vancomycin (VAN) highly resistant Staphylococcus aureus (VRSA) strains from clinical methicillin-resistant S. aureus strains by repeating steps of in vitro mutagenesis and VAN selection. Here we describe that the in vitro susceptibility of these VRSA strains to VAN was markedly increased by combined treatment with β-lactams such as ceftriaxone and oxacillin. Furthermore, in an in vivo silkworm infection model with VRSA, a combination of VAN and ceftriaxone exhibited therapeutic effects, whereas a combination of VAN and oxacillin did not. These findings suggest that combining VAN with an appropriate β-lactam, such as ceftriaxone, is therapeutically effective against infectious diseases caused by VRSA.
Similar content being viewed by others
Introduction
Vancomycin (VAN) is widely applied clinically to treat infection by methicillin-resistant Staphylococcus aureus (MRSA). The emergence of VAN-intermediate-resistant S. aureus (VISA) strains that exhibit weak tolerance against VAN (MIC: 4–8 μg ml−1) was reported recently.1 VRSA, which is highly resistant to VAN because of an exogenous gene, such as vanA, was also reported.2, 3 Teicoplanin, arbekacin, linezolid and daptomycin are considered to be therapeutically effective against VAN-resistant bacteria. Strains resistant to these antibiotics have already manifested,4, 5, 6, 7 however, and thus there is an urgent need to establish novel treatment strategies effective against infection by VAN-resistant strains.
Recently, we established a method to obtain MRSA strains that are highly resistant to VAN from clinical isolates of MRSA strains by repeating steps of mutagenesis and VAN selection.8 VRSA strains isolated using this procedure accumulated multiple genetic mutations that led to increased cell wall thickness and tolerance against high concentrations of VAN (⩾16 μg ml−1).8 VR7, which is a VRSA strain, also showed daptomycin resistance.8 We consider that these VRSA strains will be useful for establishing effective treatment strategies against emerging VAN-resistant bacteria.
Some reports suggested that combinations of VAN and β-lactams are effective against VISA.9, 10 The MIC values of VAN against VISA used in these studies, however, were relatively low (MIC: 4–8 μg ml−1),9 and thus an increased susceptibility of VISA to VAN by β-lactam was not clearly demonstrated. We considered that VRSA isolated by our method8 would be a useful tool for testing the effectiveness of a combination of VAN and β-lactams. We previously reported the synergistic effects of VAN and cefazolin or oxacillin (OXA) against VRSA strains in vitro.8 In this paper, we describe the synergistic effects of VAN and other β-lactams.
Animal models of disease are used to evaluate the therapeutic effects of drug candidates. We established an infection model using silkworms to evaluate the therapeutic effects of drug candidates against bacteria or true fungi pathogenic to humans.11, 12, 13, 14 The silkworm model is expected to be superior as an in vivo evaluation system for drug candidates to examine their therapeutic effectiveness against infectious diseases.15, 16, 17, 18, 19, 20, 21 In this paper, we describe our in vivo evaluation of the therapeutic effects of the combination of VAN and β-lactams against VRSA using the silkworm infection model.
Materials and methods
Bacterial strains and culture conditions
The bacterial strains used in this study are shown in Table 1. Eight MRSA strains (MR1–8) were isolated from human patients at the University of Tokyo Hospital.8 VAN-resistant strains (VR1–8) isolated from MRSA strains (MR1–8) by repeating steps of mutagenesis and antibiotic selection8 were used in the experiments (Table 1). Mu50 is a VISA.22 Bacteria were cultured at 37 °C in Tryptic soy broth according to the previously described method.8
Measurement of anti-bacterial activity
The MIC values of various antibiotics were determined by the microdilution method. Colonies of each strain cultured on an agar plate were scraped, suspended in sterilized saline and the OD600 was adjusted to 0.5. The samples were diluted to 1/400 in Mueller–Hinton broth supplemented with Ca2+ and Mg2+; a 190 μl bacterial suspension was dispensed in the top well of a 96-well round bottom plate and 100 μl suspensions were dispensed in the other wells. Solutions (10 μl) containing test samples were added to the top well, mixed and serially diluted twofold. After 48 h incubation, bacterial growth in each well was measured as described previously.8 A more than fourfold difference in the MIC values was judged to be significant.
Silkworm rearing
Silkworm larvae were bred according to Kaito et al.11 Briefly, eggs were purchased from Ehime Sanshu (Honai, Ehime, Japan), hatched in an incubator at 27 °C and bred. Artificial feed, Silkmate 2S, was purchased from Nihon Nosan (Yokohama, Kanagawa, Japan). Fifth instar larvae were used in the infection experiments.
Evaluation of the therapeutic effects of antibiotics using the silkworm infection model
Evaluation of the therapeutic effects of antibiotics using the silkworm infection model was performed according to Hamamoto et al.13 with some modification. Fifth instar larvae were fed artificial food (1.1–1.2 g, antibiotic free; Nihon Nosan) and bred for 20 to 24 h at 27 °C. Fifty microliters of a twofold diluted overnight culture of S. aureus (injected bacterial numbers were 1.1 × 108 per larva) was injected into the silkworm hemolymph, followed by injection of 50 μl of sterilized saline or various concentrations of antibiotics. The concentration of the injected antibiotics was as follows: VAN 100 μg ml−1, ceftriaxone (CTRX) 800 μg ml−1 and OXA 800 μg ml−1. After being injected with the bacterial culture and antibiotics, the silkworms were reared at 37 °C, and silkworm survival was monitored. The log-rank test was performed for statistical processing using the Prism software (GraphPad Software, La Jolla, CA, USA).
Results
Synergistic effects of VAN and β-lactams on the growth inhibition of VRSA
We previously reported that OXA decreased the MIC values of VAN against VRSA (VR3 and VR7).8 The concentration of OXA needed for the effect was much lower than the MIC value of OXA against the VRSA strains, indicating that the actions of OXA and VAN are synergistic.8 In the present study, we first examined whether the synergistic effects of OXA and VAN were also observed for six other VAN-resistant strains. The MIC values of OXA against these VAN-resistant strains were higher than 128 μg ml−1. In the presence of OXA 2 μg ml−1 (1/50 MIC), the MIC of VAN against the VAN-resistant strains was reduced to ⩽1/8 (Table 2). We next tested whether β-lactams other than OXA exhibited synergistic effects against VRSA (VR7). The results indicated that all nine tested β-lactams, including cefazolin and OXA, markedly decreased the MIC values of VAN against VR7 (MIC=32 μg ml−1→2–4 μg ml−1; Table 3). We checked that the concentrations (2 μg ml−1) of the nine tested β-lactams were lower than their MIC values against VR7 (Table 4). The results suggested that the synergistic effect with VAN is a general characteristic of β-lactams.
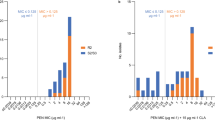
To obtain synergistic effectiveness by combined therapy with two drugs, the pharmacokinetic parameters of the two compounds, such as the half-life in blood, should be consistent.23, 24, 25 The half-lives of β-lactams including OXA are relatively short (0.4–2 h),26, 27 whereas the half-life of VAN (4–8 h)28 is much longer. On the other hand, the half-life of CTRX in human blood is relatively long (6–9 h).29 Therefore, we next examined the synergistic effects of CTRX and VAN against the VRSA strains. Our findings indicated that the MIC values of VAN against the VRSA strains were reduced by 4 μg ml−1 CTRX (Table 5). CTRX concentrations higher than 1 μg ml−1 markedly decreased the MIC value of VAN against VR7 (Figure 1). All eight VAN-resistant strains were resistant to CTRX (MIC>128 μg ml−1), suggesting that CTRX and VAN have synergistic activities.
Therapeutic effect of VAN combined with a β-lactam in the silkworm infection model with VRSA
Experiments with animal models are important for evaluating the therapeutic effects of antibiotics. We tested whether the combination of VAN and β-lactams exhibited therapeutic effects against the VRSA strains in the silkworm infection model. VRSA strains were injected into the silkworm hemolymph followed by injection of VAN and β-lactam, and survival of the animals was monitored. Survival was not extended in silkworms injected with VAN, OXA or CTRX alone (Figure 2). Silkworms injected with a combination of VAN and CTRX survived much longer than those injected with either VAN or CTRX alone (P<0.0001) (Figure 2). On the other hand, the combination of VAN and OXA did not extend the survival of the silkworms (Figure 2). In this experiment, we observed that the injection of saline or antibiotic did not cause death in non-infected silkworms.
Therapeutic effects of combined vancomycin (VAN) and ceftriaxone (CTRX) in the silkworm infection model with VAN highly resistant Staphylococcus aureus (VRSA). Survival of silkworms infected with VR7 after injection of VAN, OXA or CTRX alone or with the combinations of VAN and OXA or VAN and CTRX is shown. The number of injected bacteria was 1.1 × 108 colony-forming unit (CFU) per larva; n=5 per group.
Discussion
In this paper, we revealed that β-lactams (OXA, CTRX, cefazolin, methicillin, ampicillin, benzylpenicillin, carbenicillin, cephalexin and cloxacillin) increased the susceptibility of VRSA strains to VAN. We also demonstrated that VAN combined with CTRX exhibited therapeutic effects in the silkworm infection model with VRSA. The combination of VAN and OXA, however, exhibited no therapeutic effects in the silkworm infection model. These results suggest that combined treatment with VAN and an appropriate β-lactam is therapeutically effective against VRSA, which is expected to be a serious threat to humans.
β-Lactam antibiotics have anti-bacterial activity by associating with the penicillin-binding proteins (PBPs) of S. aureus and inhibiting the formation of the crosslinked structure between sugar chains, resulting in the inhibition of peptidoglycan biosynthesis.30 MRSA strains contain SCCmec regions that have the mecA gene coding the PBP2′, whose affinity for β-lactam is much lower than that of other PBPs.31, 32, 33 By expressing PBP2′, MRSA strains become resistant to β-lactams. Both OXA and CTRX, at concentrations at which they exhibited no anti-bacterial effects alone, 0.125 μg ml−1 (ref. 8) and 1 μg ml−1, respectively, markedly decreased the MIC values of VAN against VRSA strains. The precise mechanism underlying the synergistic effects of VAN and β-lactams is unclear. We assume that β-lactams affect cell wall synthesis at low concentrations by binding to PBPs other than PBP2′ of VRSA. VAN inhibits the peptide crosslinking reaction by binding to the d-alanine-d-alanine of the GlcNAc-MurNAc unit end.30 Therefore, we consider that a change in the cell wall structure of VRSA caused by the binding of β-lactams to PBPs could induce efficient actions of VAN, resulting in the inhibition of VRSA growth. Further studies will be needed to elucidate the detailed molecular mechanisms of the effects of β-lactams against VRSA.
We found that the MIC value of VAN against Mu50 strain, which is a clinical strain of VISA, was decreased by the addition of OXA (Tables 2 and 5). The genetic background of Mu50 and VRSA strains used in this study is different from the clinically isolated VRSA strains, which have vanA gene. Further studies will be needed to elucidate the synergistic effects of VAN and β-lactams against the clinically isolated VRSA.
Here we revealed that combinations of all nine β-lactams examined, including OXA, exhibited clear synergistic effects with VAN against the in vitro growth of VRSA (Table 1). On the other hand, in the in vivo evaluation of drug efficacy in silkworms, the combination of OXA and VAN exhibited no therapeutic effects (Figure 2). In humans, the half-life of OXA in the blood is very short, and binding rates to serum proteins are very high, which complicates the pharmacokinetics.27 We consider that the half-life of OXA may be also short in the silkworm hemolymph, resulting in a loss of therapeutic effectiveness in combination with VAN. Further studies using mouse infection models treated by humanized doses of antibiotics will be needed, to evaluate the combination taking into account the respective pharmacokinetic profile of VAN and β-lactams.
Several reports have suggested the effectiveness of the combination of VAN and β-lactams against MRSA.34, 35, 36 Because the MIC values of VAN against MRSA are relatively low (⩽2 μg ml−1), it is unclear whether the MIC values of MRSA are decreased by β-lactams. In this study, we used VRSA that is highly resistant to VAN, and demonstrated that β-lactams decreased the MIC value of VAN against these VRSA strains. In the experiments we performed at the same time with VRSA, most of the combinations of VAN and β-lactams were effective against MRSA. Recently, a phenomenon called ‘MIC creep’, that is, an increase in the MIC value of VAN against MRSA, was reported in patients.37 Our results support the notion that combined treatment with VAN and β-lactams might be an effective strategy against the MIC creep.
The concept of ‘drug repositioning’ in which the application of existing drugs is expanded to treat various diseases has been proposed.38, 39 Extending the idea of drug repositioning, we propose a new concept of ‘drug reuse’. Key to this concept is the combined use of antibiotics that are judged to be unsuitable by themselves due to the emergence of drug-resistant bacteria. Because the safety of individual antibiotics has already been established, combinations of these drugs with sufficient evidence for their clinical efficacy have attracted attention as novel treatments against drug-resistant bacteria. For example, the combination of sulfametoxazol with trimethoprim, two types of folic acid metabolism antagonists, exhibits clinical effects.40 The development of new therapies by combined treatment with antibiotics, such as VAN and β-lactams, whose safety and pharmacokinetics are well established, will be useful to overcome the problems associated with the emergence of drug-resistant bacteria.
References
Howden, B. P., Davies, J. K., Johnson, P. D., Stinear, T. P. & Grayson, M. L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23, 99–139 (2010).
Chang, S. et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348, 1342–1347 (2003).
Melo-Cristino, J., Resina, C., Manuel, V., Lito, L. & Ramirez, M. First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. Lancet 382, 205 (2013).
Robert, J., Bismuth, R. & Jarlier, V. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983-2002. J. Antimicrob. Chemother. 57, 506–510 (2006).
Ishino, K., Ishikawa, J., Ikeda, Y. & Hotta, K. Characterization of a bifunctional aminoglycoside-modifying enzyme with novel substrate specificity and its gene from a clinical isolate of methicillin-resistant Staphylococcus aureus with high arbekacin resistance. J. Antibiot. (Tokyo) 57, 679–686 (2004).
Wilson, P. et al. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51, 186–188 (2003).
Mehta, S. et al. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56, 92–102 (2012).
Ishii, K. et al. Phenotypic and genomic comparisons of highly vancomycin-resistant Staphylococcus aureus strains developed from multiple clinical MRSA strains by in vitro mutagenesis. Sci. Rep. 5, 17092 (2015).
Werth, B. J. et al. Novel combinations of vancomycin plus ceftaroline or against methicillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA. Antimicrob. Agents Chemother. 57, 2376–2379 (2013).
Domenech, A. et al. Experimental study on the efficacy of combinations of glycopeptides and beta-lactams against Staphylococcus aureus with reduced susceptibility to glycopeptides. J. Antimicrob. Chemother. 56, 709–716 (2005).
Kaito, C., Akimitsu, N., Watanabe, H. & Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32, 183–190 (2002).
Matsumoto, Y. et al. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 112, 138–146 (2012).
Hamamoto, H. et al. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 48, 774–779 (2004).
Uchida, R., Namiguchi, S., Ishijima, H. & Tomoda, H. Therapeutic effects of three trichothecenes in the silkworm infection assay with Candida albicans. Drug Discov. Ther. 10, 44–48 (2016).
Usui, K. et al. Acute oral toxicity test of chemical compounds in silkworms. Drug Discov. Ther. 10, 57–61 (2016).
Fujiyuki, T., Imamura, K., Hamamoto, H. & Sekimizu, K. Evaluation of therapeutic effects and pharmacokinetics of antibacterial chromogenic agents in a silkworm model of Staphylococcus aureus infection. Drug Discov. Ther 4, 349–354 (2010).
Hamamoto, H. et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat. Chem. Biol. 11, 127–133 (2015).
Uchida, R. et al. In vitro and in vivo anti-MRSA activities of nosokomycins. Drug Discov. Ther. 8, 249–254 (2014).
Uchida, R. et al. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. I: fermentation, isolation and biological properties. J. Antibiot. (Tokyo) 63, 151–155 (2010).
Tomoda, H. New approaches to drug discovery for combating MRSA. Chem. Pharm. Bull. (Tokyo) 64, 104–111 (2016).
Uchida, R., Iwatsuki, M., Kim, Y. P., Omura, S. & Tomoda, H. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. II: structure elucidation. J. Antibiot. (Tokyo) 63, 157–163 (2010).
Hiramatsu, K. et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350, 1670–1673 (1997).
Odawara, M., Hamada, I. & Suzuki, M. Efficacy and safety of vildagliptin as add-on to metformin in Japanese patients with type 2 diabetes mellitus. Diabetes Ther. 5, 169–181 (2014).
He, Y. L. et al. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin. Pharmacokinet. 46, 577–588 (2007).
Kajbaf, F., Bennis, Y., Hurtel-Lemaire, A. S., Andrejak, M. & Lalau, J. D. Unexpectedly long half-life of metformin elimination in cases of metformin accumulation. Diabet. Med 33, 105–110 (2016).
Lee, F. H., Pfeffer, M., Van Harken, D. R., Smyth, R. D. & Hottendorf, G. H. Comparative pharmacokinetics of ceforanide (BL-S786R) and cefazolin in laboratory animals and humans. Antimicrob. Agents Chemother. 17, 188–192 (1980).
Barza, M. & Weinstein, L. Some determinants of the distribution of penicillins and cephalosporins in the body. Practical and theoretical considerations. Ann. NY Acad. Sci. 235, 613–620 (1974).
Van Bambeke, F., Van Laethem, Y., Courvalin, P. & Tulkens, P. M. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 64, 913–936 (2004).
Lamb, H. M., Ormrod, D., Scott, L. J. & Figgitt, D. P. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 62, 1041–1089 (2002).
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000).
Katayama, Y., Ito, T. & Hiramatsu, K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44, 1549–1555 (2000).
Hartman, B. J. & Tomasz, A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158, 513–516 (1984).
Ubukata, K., Nonoguchi, R., Matsuhashi, M. & Konno, M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J. Bacteriol. 171, 2882–2885 (1989).
Komatsuzawa, H., Suzuki, J., Sugai, M., Miyake, Y. & Suginaka, H. Effect of combination of oxacillin and non-beta-lactam antibiotics on methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 33, 1155–1163 (1994).
Domaracki, B. E., Evans, A. M. & Venezia, R. A. Vancomycin and oxacillin synergy for methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 44, 1394–1396 (2000).
Drago, L., De Vecchi, E., Nicola, L. & Gismondo, M. R. In vitro evaluation of antibiotics’ combinations for empirical therapy of suspected methicillin resistant Staphylococcus aureus severe respiratory infections. BMC Infect. Dis. 7, 111 (2007).
Steinkraus, G., White, R. & Friedrich, L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J. Antimicrob. Chemother. 60, 788–794 (2007).
Ashburn, T. T. & Thor, K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 (2004).
Mizushima, T. Drug discovery and development focusing on existing medicines: drug re-profiling strategy. J. Biochem. 149, 499–505 (2011).
Bushby, S. R. & Hitchings, G. H. Trimethoprim, a sulphonamide potentiator. Br. J. Pharmacol. Chemother. 33, 72–90 (1968).
Acknowledgements
This project was supported by JSPS KAKENHI grant number JP15H05783 (Scientific Research (S) to KS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
KS has an advisory role at Genome Pharmaceuticals Institute (Tokyo, Japan). The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tabuchi, F., Matsumoto, Y., Ishii, M. et al. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J Antibiot 70, 771–774 (2017). https://doi.org/10.1038/ja.2017.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.7
This article is cited by
-
In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus
Scientific Reports (2021)
-
Evidence from an In Vitro Study: Is Oxacillin Plus Vancomycin a Better Choice for Heteroresistant Vancomycin-Intermediate Staphylococcus aureus?
Infectious Diseases and Therapy (2019)
-
D-cycloserine increases the effectiveness of vancomycin against vancomycin-highly resistant Staphylococcus aureus
The Journal of Antibiotics (2017)