Abstract
Tunicamycin is a Streptomyces-derived inhibitor of eukaryotic protein N-glycosylation and bacterial cell wall biosynthesis, and is a potent and general toxin by these biological mechanisms. The antibacterial activity is dependent in part upon a π-π stacking interaction between the tunicamycin uridyl group and a specific Phe residue within MraY, a tunicamycin-binding protein in bacteria. We have previously shown that reducing the tunicamycin uridyl group to 5,6-dihydrouridyl (DHU) significantly lowers its eukaryotic toxicity, potentially by disrupting the π-stacking with the active site Phe. The present report compares the catalytic hydrogenation of tunicamycin and uridine with various precious metal catalysts, and describe optimum conditions for the selective production of N-acyl reduced tunicamycin or for tunicamycins reduced in both the N-acyl and uridyl double bonds. At room temperature, Pd-based catalysts are selective for the N-acyl reduction, whereas Rh-based catalysts favor the double reduction to provide access to fully reduced tunicamycin. The reduced DHU is highly base-sensitive, leading to amide ring opening under mild alkaline conditions.
Similar content being viewed by others
Introduction
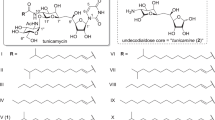
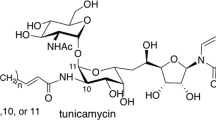
Tunicamycin is a potent and selective inhibitor of the UDP-N-acetyl-d-hexosamine:isoprenol-1-phosphate UDP-HexNAc-1-P translocase (PNPT) family of enzymes, and is widely used to block the first step in eukaryotic protein N-glycosylation or of the assembly of bacterial cell walls.1, 2, 3, 4 It consists of an 11-carbon dialdose sugar, tunicamine, which is N-glycosidically linked to uracil, and αβ-1,11-O-glycosidically linked to N-acetylglucosamine (Figure 1). Several Streptomyces species produce tunicamycins, notably S. chartreusis and S. lysosuperificus,5, 6 although other strains are known to produce related compounds, such as streptovirudins, corynetoxins, and quinovosamycins.7, 8, 9, 10 Tunicamycin biosynthesis has been studied in S. chartreusis NRRL B-3882 and showed that the 11-carbon tunicamine dialdose is derived from ligation of 6-carbon pyranose and 5-carbon furanose units, the latter derived from uridine or ribose.11, 12, 13, 14 This suggested that there may be an active uridine salvage pathway in the tunicamycin-producing S. chartreusis. Most of the known tunicamycin-related compounds contain an N-glycosidically linked uridyl group that acts as a substrate analog of the nucleotide on the UDP-HexNAc donor substrate of PNPT. However, several of the streptovirudins isolated from S. griseoflavus subsp. thuringiensis have related structures to the tunicamycins except that the uridyl group is replaced by 5,6-dihydrouracyl (Figure 1).7
Recent work by Hakulinen et al.15 has shown that tunicamycin binds at a cytoplasmic cavity of MraY, a bacterial PNPT transmembrane protein. Significantly, the tunicamycin uracil ring is shown to bind to a uridyl binding pocket within the MraY protein, composed of Gly-176, Asn-221, and Phe-228. Other essential residues, Asp-175, Glu-300, and Lys-74 are adjacent to the 2-carbonyl of the uracil ring.15 This tunicamycin uracil binding pocket (UBP) shares a partial overlapping binding mode also found for the uracil group of muraymycin D, a related nucleotide inhibitor of MraY.16, 17 Comparable π-π stacking of Phe-228 occurs with both tunicamycin and muraymycin, and also with a truncated MraY substrate, UDP-MurNAc-L-Ala, providing a mechanism for the binding of the tunicaminyl uracil motif.15, 17
The present work investigates the catalytic hydrogenation of tunicamycin, in an attempt to selectively reduce the N-acyl and/or uridyl double bonds on a preparative scale (Scheme 1). We report that hydrogenation at ambient temperature and pressure with Pd/C catalyst results in selective reduction of the conjugated 2′′′, 3′′′-N-acyl double bond to give Tun-R1. Substituting Rh/Al2O3 catalyst results in the reduction of both double bonds to give Tun-R2. Moreover, the reduced uridyl ring is shown to be is highly sensitive to base-catalyzed ring opening. We have previously shown that Tun-R1 and Tun-R2 both exhibit considerably reduced eukaryotic toxicity when compared to the tunicamycin natural products.18
Results
Selective catalytic hydrogenation of the tunicamycin uridyl and N-acyl double bonds
Methods have been investigated for the selective chemical reduction of the tunicamycins. Reduction of the tunicaminyl double bonds with sodium borohydride in the presence of Pd/C catalyst, although successful on a small scale, was complicated by the post reaction removal of borate and acetate co-products.18 Moreover, unless the pH is strictly controlled the borohydride treatment can lead to an undesirable uridyl ring opening.19, 20, 21 Kochetor et al.22 have described catalytic hydrogenation of uridine over Rh/Al2O3 catalyst in aqueous solution requiring the use of lithium acetate buffer, which requires subsequent removal. Using uridine as a model for the tunicamycin uridyl motif we obtained complete reduction to 5,6-dihydrouridine in methanol using Rh/Al2O3-catalyzed hydrogenation at 1 atm. pressure and room temperature. The reaction progress was monitored by MALDI-TOF MS by following the conversion of the [M+H]+ and [M+Na]+ ions at m/z 245.2 and 267.2 (for uridine) to m/z 247.2 and 269.2 (for dihydrouridine) (Figures 2a and b). Following a straightforward recovery by centrifugation or filtration as needed to remove spent catalyst, the product was characterized by NMR (Figures 2c and d and Supplementary Data). The uridyl H5 and H6 methine protons (at 5.8 and 7.8 p.p.m., respectively) are entirely absent after 3 h reaction, with concomitant generation of new methylene signals at 3.5 and 3.7 p.p.m. (Figure 2d and Table 1). These latter two signals and the adjacent ribosyl H1′ integrate to 2:2:1, indicating a clean reduction of the double bond without degradation or racemization of the uridyl motif. The dihydrouridine product was further characterized by 13C-NMR, DEPT, COSY, HSQC, HMBC, and NOESY NMR (Table 1; Supplementary Data). The HMBC experiment was crucial in showing that the carbonyl 13C signals (154.6 and 173.8 p.p.m.) are not reduced during the hydrogenation.
Hydrogenation of uridine with Pd/C (a, c) or Rh/Al2O3 (b, d) catalyst. (a, b) MALDI-TOF MS showing [M+H]+ and [M+Na]+ ions for uridine (a) and 5,6-dihydrouridine (b), respectively. m/z 273.2 is a MALDI-MS matrix ion. (c, d) 1H-NMR shows quantitative reduction of the uridyl 5, 6-double bond after hydrogenation with the Rh/Al2O3 catalyst (d).
Similar results were obtained on uridine using Rh/C catalyst in place of the Rh/Al2O3. However, with Pd/C no reduction of the uridyl ring occurred (Figure 3c). This suggested a selective reduction scheme for tunicamycin, whereby Pd/C-catalyzed hydrogenation is used to selectively reduce the N-acyl double bond, and Rh/Al2O3-catalyzed hydrogenation for double reduction of both N-acyl and uridyl double bonds (see Figure 1 for the tunicamycin structure). This was investigated by monitoring the hydrogenation of tunicamycin under these selective conditions by MALDI-TOF MS (Figure 3), and by proton NMR analysis of the double bond region (5–8 p.p.m.; Figure 4).
MALDI-TOF MS analysis of tunicamycins (a), and after catalytic hydrogenation with Pd/C (b) or Rh/Al2O3 (c) catalysts. (a). Tun-14:1, m/z 839.8; Tun-15:1, m/z 853.8; Tun-16:1, m/z 867.9; Tun-17:1, m/z 881.9. (b). Tun-14:0, m/z 841.8; Tun-15:0, m/z 855.8; Tun-16:0, m/z 869.9; Tun-17:0, m/z 883.9. (c) DHU-Tun-14:0, m/z 843.7; DHU-Tun-15:0, m/z 857.7; DHU-Tun-16:0, m/z 871.7; DHU-Tun-17:0, m/z 885.8. The MS spectra were acquired in reflectron mode using 2,5-dihydroxybenzoic acid as matrix.
(i) Proton NMR spectra (5.6 – 8.2 p.p.m. region) of tunicamycin after hydrogenation with either Pd/C (a–c) or Rh/Al2O3 catalysts (d–f). Timed reactions with H2 gas (1 atm. in methanol) were stopped at 30 min (a, d), 1 h (b, e) or 3 h (c, f). Signals arise from the starting tunicamycin (uridyl ring; H5, 5.77 p.p.m.; H6, 7.91 p.p.m.; pseudoribosyl anomeric H1′(U), 5.90 p.p.m.; N-acyl H2′′′, 5.95 p.p.m.; H3′′′, 6.83 p.p.m.); the N-acyl-reduced tunicamycin, TunR1 (uridyl ring; H5, 5.77 p.p.m.; H6, 7.91 p.p.m.; pseudoribosyl anomeric H1′(U), 5.90 p.p.m.); and the fully reduced dihydrotunicamycin, TunR2 (pseudoribosyl anomeric H1′(d), 5.88 p.p.m.). (ii) HMBC NMR spectrum of the four carbonyl correlation for the dihydrouridyl-tunicamycin produced by Rh/Al2O3-catalyzed hydrogenation. The 13C carbonyl signals are (A). uridyl-2; (B). uridyl-4; (C). N-acetyl; (D). N-acyl. The associated 1H signals are assigned in Table 1.
MALDI-TOF MS peaks at m/z 853.8, 867.9, and 881.9 correspond to the [M+Na]+ adduct ions for Tun-15:1, Tun16:1, and Tun17:1, tunicamycin components with N-pentadecenoate, N-hexadecenoate and N-heptadecenoate N-acyl groups, respectively (Figure 3a). The single reduction of these molecules is evident from ions at m/z 855.8, 869.9 and 883.9, and the double reduction by m/z 857.7, 871.7 and 885.8, mass increases of two or four protons, respectively (Figures 3b and c). However, these data are not selective for the position of the reductions. This required a quantitative analysis of the uridyl H5 and H6 double bond protons at 7.91 p.p.m. and 5.77 p.p.m. for the redox state of the uridyl group, and at 6.83 and 5.95 p.p.m. for the N-acyl double bond (Figure 4 (i); Table 1). The integrals of these signals indicate that after 30 min hydrogenation with Pd/C catalyst that the uridyl H5/H6 double bond is still intact, and that the H5/H6 uridyl protons and the tunicaminyl pseudoribosyl αH1′ signal (5.90 p.p.m.) integrate to 1:1:1 (Figure 4 (i)). Noticeably, the N-acyl double bond is completely reduced by this time point, with the loss of the H2′′′ and H3′′′ methine signals at 6.85 and 5.95 p.p.m. At 3 h there is also a 70% attenuation of the uridyl H5/H6 protons relative to the pseudoribosyl αH1′, indicating about a 70% conversion to dihydrouridyl (Figure 4 (i)). This is also evident from the chemical shift of the pseudoribosyl αH1′ on the reduced dihydrouridyl-tunicamycin, which now appears at 5.88 p.p.m. (Figure 4 (i)). These data show that the Pd/C catalyst is selective toward the tunicamycin N-acyl group in methanol at room temperature.
An analogous series of reactions was done on tunicamycin with Rh/Al2O3 catalyst using methanol as the solvent. After 30 min reaction time the N-acyl double bond 1H-NMR signals are still present, but reduced by ~90%, indicating that the reduction of the N-acyl group is not completed by this time (Figure 4 (i)). This is in contrast to that observed with the Pd/C catalyst, which gave complete reduction of the N-acyl double bond by 30 min. However, unlike on Pd/C, the tunicamycin uridyl group is also partially hydrogenated by 30 min with the Rh/Al2O3 catalyst (Figure 4 (i)). This is evident from the attenuated integrals of H5/H6 relative to αH1′, and from the de novo αH1′ of the dihydrouridyl-pseudoribosyl at 5.88 p.p.m. The relative integrals of these signals show that the uridyl group is ~30% reduced by 30 min with the Rh/Al2O3 catalyst (Figure 4 (i)). After 1 h the reduction of both double bonds is apparently completed, and with no evidence of further modification after 3 h. This double-reduced tunicamycin product was readily recovered by filtering off the catalyst, and was fully characterized by COSY, HSQC, TOCSY, 13C, and HMBC NMR (Supplementary Data). The HMBC spectra of the high field region indicated that the four carbonyls (154.1, 171.6, 172.2, and 175.8 p.p.m.) in the dihydrouridyl-tunicamycin are still intact (Figure 4 (ii)) as observed previously with the uridine model compound. The HMBC signal at 154.1 p.p.m. is assigned as the dihydrouridyl ring C2 carbonyl, and is seen to correlate with the pseudoribosyl αH1′ signal (5.90 p.p.m.) and the reduced H6a and H6b signals at 3.5 – 3.6 p.p.m. The C4 carbonyl in the dihydrouridyl ring is assigned at 171.6 p.p.m., and also correlates to the H6a and H6b protons, plus the reduced H5 CH2 at 2.70 p.p.m. The N-acetyl and N-acyl carbonyls are assigned at 172.2 p.p.m. and 175.8 p.p.m., respectively, and show correlations to the N-acetyl methyl signal at 2.03 p.p.m., or the N-acyl α-CH2 signals at 2.21 p.p.m. Moreover, a comparison of the HSQC, 1H, and 13C NMR spectra of the Rh/Al2O3-catalyzed hydrogenation product with dihydrouridyl-tunicamycin (Tun R2) produced by 50 h at reflux with NaBH4/Pd/C showed them to be identical (Supplementary Data), although the catalytic hydrogenation is achieved in 3 h and without the required post-reaction cleanup of borate salts.
Photoreduction and alkaline lability of the dihydrouridyl group
Cerutti et al.23 reported in 1965 that photoexcitation of certain heterocyclic compounds enhances their reduction by sodium borohydride, and used this to selectively photoreduce uridine in RNA polynucleotides. As part of this work they also reported a preparative reaction for the selective photoreduction of uridine to 5,6-dihydrouridine.24 More recently, the photoreduction of carbonyls with borohydride has been reported,25 and others found that UV irradiation of polyunsaturated allylic benzoates results in a selective E/Z interconversion of the allylic double bond.26 This suggested a possible selective photoreduction of the uridyl group of tunicamycins without the attendant reduction of the N-acyl double bond which, if successful, might be an expedient route for the chemical conversion of tunicamycins into streptovirudins (Figure 1). However, our attempts to replicate the photoreduction of uridine under Cerutti conditions were unsuccessful. House and Miller found that the dihydrouridine residue found in tRNA is stable at 25 °C, but with a short half-life at higher temperatures and pH leading to a ring opening of the dihydrouridine to ureidopropanol riboside.19 Cleavage of the dihydrouridine ring has also been reported at mild alkaline conditions and upon reduction by sodium borohydride. In both cases, cleavage of the dihydrouridine ring is followed by cleavage of the RNA chain (Behm-Ansmant et al,27).
To investigate the pH stability of the dihydrouridine group we acquired in situ NMR spectra of the dihydrouridine prepared by Rh/Al2O3-catalyzed hydrogenation of uridine in the presence of either deuterated NaOD or CF3COOD (Figure 5). The DHU is stable under acid condition, and the NMR data were unchanged over a period of 18 h (Figure 5b). However, in the presence of the NaOD the dihydrouridine was quantitatively hydrolyzed to ureidopropanol riboside, without any cleavage of the adjacent ribosyl N-glycosidic bond (Figure 5c). This result was confirmed when dihydrouridine was treated with 0.25M sodium hydroxide for 18 h at ambient temperature and analyzed by MALDI TOF mass spectrometry (data not shown). The molecular ions for DHU ([M+H]+, m/z 247.2; [M+Na]+, m/z 269.2) were increased by 18 Da to m/z 265.2 and m/z 287.2 by the hydrolysis of the cyclic peptide bond.
Discussion
The uridyl structural motif is a fairly widespread chemophore for a number of natural products.28, 29 However, the 5,6-dihydrouridyl group is less common. Mureidomycins, napsamycins, and streptovirudins have dihydrouracil analogs (Figure 1), and these compounds all target the bacterial MraY members of the PNPT enzyme family. For the napsamycins a biosynthetic gene, npsU, has been identified that encodes a putative reductase that is responsible for the reduction of the uridyl group to dihydrouridyl.30 A related group of 5,6-dihydrouridyl-containing nucleotides includes jawsamycin (FR-900848) from Streptoverticillium fervens (Figure 1),31 the sesquiterpenoid nucleotide esters farnesides A and B from Streptomyces sp. CNT-372,32 and an anti-influenza virus compound called JBIR-68 from Streptomyces sp. RI18.33 Moreover, a putative reductase gene. jaw1, has been identified in Streptoverticillium fervens that is responsible for the reduction of dehydrojawsamycin to jawsamycin.34 Noticeably, although jaw1 and npsU are both classified as PPOX class F-420-dependent reductases, and they are both involved in the reduction of uridyl double bonds, there is very low homology between these two genes,34 and their use for the prediction of genes involved in the potential reduction of tunicamycins is therefore not viable. Catalytic hydrogenation and photoreduction methods were therefore investigated for the potentially selective chemical reduction of tunicamycins. Photoinduced reductions were unsuccessful, but the use of selected hydrogenation catalyst (either Pd or Rh) are shown to selectively reduce the double bonds of tunicamycins. Hence, this provides a straightforward way to scale up the preparation of reduced toxicity tunicamycin analogs, Tun-R1 and Tun-R2, for further biological testing.
Materials and methods
Materials
Tunicamycin was purchased from Alfa Aesar, Ward Hill, MA. 5 wt% Pd/C was from the Calsicat division of Mallinkrodt Chemical, Erie, PA and the 5 wt% Rh/Al2O3 was from BASF Engelhard, Iselin, NJ. All other chemicals, including the MALDI-TOF MS matrix, were obtained from Sigma-Aldrich Inc., St Louis, MO, USA.
Analytical techniques
Matrix-assisted laser desorption/ionization time-of-flight mass spectra (MALDI-TOF MS) were acquired in a Bruker-Daltonics Microflex instrument (Bruker-Daltonics, Billerica, MA, USA) running in reflectron mode. Ion source 1 and 2 were set to 19.0 and 14.0 kV, respectively, with lens and reflector voltages of 9.20 and 20.00 kV. The laser (337.1 nm) was typically at 60% of 150 μJ maximum output, and 3000 shots are accumulated. Samples under analysis were dissolved in methanol:water (50:50 v/v, 5 μl) prior to co-crystallization with the matrix. The matrix used was 2,5-dihydrobenzoic acid. NMR spectra were obtained on a Bruker Avance III instrument (Bruker BioSpin, Billerica, MA, USA) operating at 500.11 Mhz using a 5 mm z-gradient BBI probe at 27 ºC. The samples under analysis were dissolved in CD3OD (Sigma-Aldrich Inc.). Chemical shifts are reported as p.p.m. from TMS calculated from the lock solvent.
Hydrogenation of uridine
Uridine (500 mg) was dissolved in 32 ml methanol to which 100 mg Rh/Al2O3 were added. This suspension was placed in a Parr Instruments (Parr Instruments, Inc., Moline, IL, USA) stirred reactor using a glass liner. The reactor was purged with H2 to remove air and charged to 6.6 bar H2 pressure. The reaction was allowed to proceed at 21 °C and gave complete conversion to DHU in 6 h.
Synthesis of N-acyl reduced tunicamycin (Tun-R1)
Twenty milligrams of 5 wt% Pd/C were suspended in 3 ml methanol containing 30 mg tunicamycin. This suspension was stirred at room temperature and purged briefly with H2. The septum-capped vial was charged at 1 atm. H2 until the reaction was complete. The product was isolated by removing the catalyst by centrifugation.
Synthesis of doubly reduced tunicamycin (Tun-R2)
As described for the singly reduced tunicamycin except that 50 mg 5 wt% Rh/Al2O3 were suspended in 3 ml methanol containing 30 mg tunicamycin. The product was isolated using the same procedure as for isolation of the N-acyl reduced tunicamycin.
Photochemical reactions and base-catalyzed ring opening
Photoinduced reductions were undertaken essentially as described by Cerutti et al.24 Uridine (3 mg) and sodium borohydride (5 mg) were dissolved in 3 ml of aqueous methanol (2:1 v/v). This was allowed to spread as a thin film in a 5 ml glass Petri dish, and was irradiated open with short wavelength UV light for 2 h at a distance of 2 cm. The solution was transferred to a tube and treated with Dowex 50W H+ to destroy the excess borohydride, and analyzed by MALDI-TOF MS. For the base-catalyzed ring opening reactions, DHU was prepared by catalytic hydrogenation with Rh/Al2O3 as described, and subsequently treated for 18 h at room temperature using 0.25 M aqueous sodium hydroxide. The products were analyzed directly by MALDI TOF MS. In addition, a small scale hydrolysis was undertaken in situ in an NMR tube using 10 mg of DHU in 0.25 M sodium deuteride dissolved in deuterated water. The reaction was monitored by 1H-NMR. Control reactions were undertaken by replacing the NaOD with deuterated trifluoroacetic acid or under neutral conditions in D2O.

Selective catalytic hydrogenations of the tunicamycin antibiotics. A full colour version of this figure is available at the Journal of Antibiotics journal online.
References
Tamura, G Tunicamycin, (Japan Scientific Societies Press, Tokyo, Japan, (1982).
Heifetz, A., Keenan, R. W. & Elbein, A. D. Mechanism of action of tunicamycin on theUDP-GlcNAc: dolichyl phosphate GlcNAc-1-phosphate transferase. Biochemistry 18, 2186–2192 (1979).
Price, N. P. & Momany, F. A. Modeling bacterial UDP-HexNAc: polyprenol-P HexNAc-1-P transferases. Glycobiology 15, 29–42 (2005).
Lehrman, M. A. A family of UDP-GlcNAc/MurNAc: polyisoprenol-P GlcNAc/MurNAc-1-Ptransferases. Glycobiology 4, 768–771 (1994).
Eckardt, K Tunicamycins, streptovirudins, and corynetoxins, a special subclass of Q8 nucleoside antibiotics. J. Nat. Prod. 46, 544–550 (1983).
Doroghazi, J. R. et al. Genome sequences of three tunicamycin-producing streptomyces strains, S. chartreusis NRRL 12338, S. chartreusis NRRL 3882, and S. lysosuperificus ATCC 31396. J. Bacteriol. 193, 7021–7022 (2011).
Eckardt, K., Ihn, W., Tresselt, D. & Krebs, D. The chemical structures of streptovirudins. J. Antibiot 34, 1631–1632 (1981).
Vogel, P., Stynes, B. A., Coackley, W., Yeoh, G. T. & Petterson, D. S. Glycolipid toxins from parasitised annual ryegrass: a comparison with tunicamycin. Biochem. Biophys. Res. Commun. 105, 835–840 (1982).
Edgar, J. A. et al. Corynetoxins, causative agents of annual ryegrass toxicity; their identification as tunicamycin group antibiotics. J. Chem. Soc. Commun. 1982, 222–224 (1982).
Price, N. P. et al. Quinovosamycins: new tunicamycin-type antibiotics in which the α, β-1″,11'-linked N-acetylglucosamine residue is replaced by N-acetylquinovosamine. J. Antibiot. 69, 637–646 (2016).
Price, N. P. J. & Tsvetanova, B. C. Biosynthesis of the tunicamycins: a review. J. Antibiot 60, 485–491 (2007).
Tsvetanova, B. C., Keimle, D. J. & Price, N. P. J. Biosynthesis of tunicamycin andmetabolic origin of the 11-carbon dialdose sugar, tunicamine. J. Biol. Chem. 277, 35289–35296 (2002).
Wyszynski, F. J., Hesketh, A. R., Bibb, M. J. & Davis, B. G. Dissecting tunicamycin biosynthesis by genome mining: cloning and heterologous expression of a minimal gene cluster. Chem. Sci 1, 581–589 (2010).
Chen, W. et al. Characterization of the tunicamycin gene cluster unveiling unique steps involved in its biosynthesis. Protein Cell 1, 1093–1105 (2010).
Hakulinen, J. K. et al. MraY-antibiotic complex reveals details of tunicamycin mode of action. Nat. Chem. Biol. 13, 265–267 (2017).
Chung, B. C. et al. Crystal structure of MraY, an essential membrane enzyme for bacterial cell wall synthesis. Science 341, 1012–1016 (2013). doi:10.1038/ja.2017.101. [Epub ahead of print].
Chung, B. C. et al. Structural insights into inhibition of lipid I production in bacterial cell wall synthesis. Nature 533, 557–560 (2016).
Price, N. P. J. et al. Modified tunicamycins with reduced eukaryotic toxicity that enhance the antibacterial activity of β-lactams. J. Antibiot (e-pub ahead of print 27 September 2017; doi:10.1038/ja.2017.101.
House, C. H. & Miller, S. L. Hydrolysis of dihydrouridine and related compounds. Biochemistry 35, 315–320 (1996).
Igo-Kemenes, T. & Zachau, H. G. On the specificity of the reduction of transfer ribonucleic acids with sodium borohydride. Eur. J. Biochem. 10, 549–556 (1969).
Cerutti, P. & Miller, N. Selective reduction of yeast transfer ribonucleic acid with sodium borohydride. J. Mol. Biol. 26, 55–66 (1967).
Kochetkov, N. K., Budovskii, E. I., Shibaev, V. N. & Eliseeva, G. I. The synthesis of dihydrouridine diphosphate glucose. Russ. Chem. Bull. 14, 884–885 (1965).
Cerutti, P., Ikeda, K. & Witkop, B. The selective photoreduction of uridine in polynucleotides. J. Am. Chem. Soc. 87, 2505–2507 (1965).
Cerutti, P., Kondo, Y., Landis, W. R. & Witkop, B. Photoreduction of uridine and reduction of dihydrouridine with sodium borohydride. J. Am. Chem. Soc. 90, 771–775 (1968).
Choi, J. H., Kim, D. W. & Shim, S. C. Photo-enhanced reduction of carbonyl compounds by sodium borohydride. Tetrahedron Lett. 27, 1157–1160 (1986).
Hu, T. & Corey, E. J. A novel and selective photoisomerization of allylic benzoates. Org. Lett. 3, 3547–3548 (2001).
Behm-Ansmant, I., Helm, M. & Motorin, Y. Use of specific chemical reagents for detection of modified nucleotides in RNA. J. Nucleic Acids 2011, 1–17 (2011).
Kimura, K.-I. & Bugg, T. D. H. Recent advances in antimicrobial nucleoside antibiotics targeting cell wall biosynthesis. Nat. Prod. Rep. 20, 252–273 (2003).
Chen, W. et al. Natural and engineered biosynthesis of nucleoside antibiotics in Actinomycetes. J. Ind. Microbiol. Biotechnol. 43, 401–417 (2016).
Kaysser, L. et al. Identification of a napsamycin biosynthesis gene cluster by genome mining. Chembiochem 12, 477–487 (2011).
Yoshida, M. et al. A novel antifungal antibiotic, FR-900848. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 43, 748–754 (1990).
Zafrir Ilan, E. et al. Farnesides A and B, sesquiterpenoid nucleoside ethers from a marine-derived Streptomyces sp., strain CNT-372 from Fiji. J. Nat. Prod. 76, 1815–1818 (2013).
Takagi, M. et al. Anti-influenza virus compound from Streptomyces sp. RI18. Org. Lett. 12, 4664–4666 (2010).
Hiratsuka, T. et al. Biosynthesis of the structurally unique polycyclopropanated polyketide-nucleoside hybrid jawsamycin (FR-900848). Angew. Chem. Int. Ed. Engl. 53, 5423–5426 (2014).
Acknowledgements
We thank Trina Hartman for technical assistance, and Dr Joseph O Rich for pre-review of the manuscript. A provisional patent application (patent no. 62/450,760) has been filed. BY acknowledges the support of the National Natural Science Foundation of China (21372253 and 21432012). Mention of any trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Price, N., Jackson, M., Vermillion, K. et al. Selective catalytic hydrogenation of the N-acyl and uridyl double bonds in the tunicamycin family of protein N-glycosylation inhibitors. J Antibiot 70, 1122–1128 (2017). https://doi.org/10.1038/ja.2017.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.141
This article is cited by
-
Synergistic enhancement of beta-lactam antibiotics by modified tunicamycin analogs TunR1 and TunR2
The Journal of Antibiotics (2019)