Abstract
Previously, we discovered geldanamycin, a ligand of heat shock protein 90, effectively inhibited herpes simplex virus type 1 replication in vitro and in vivo (mouse encephalitis model). In this study, we demonstrate that geldanamycin has very strong activities against herpes simplex virus type 2 in vitro and in vivo (mouse vagina model). In mouse vagina model, administration of geldanamycin suspension to vagina after virus infection protected the infected mice from death and increased the average survival days in a dose-dependent manner. Geldanamycin also significantly reduced virus shedding from mouse vagina. All geldanamycin-treated groups were statistically significant when compared with the infected control group. The high-dose group of geldanamycin (5.72 mg kg−1) was better than acyclovir group (2.86 mg kg−1). All geldanamycin vaginal administration mock-infected groups did not show significant body weight loss. Although geldanamycin has strong antiviral activities against various DNA and RNA viruses, geldanamycin is not suitable for systemic administration because of its high toxicity. We consider that geldanamycin is a candidate of topical usage for the treatment of herpes simplex virus type infections.
Similar content being viewed by others
Introduction
Herpes simplex virus type 2 (HSV-2) is a member of the α-subfamily of human herpes viruses. HSV-2 infects the human genital tract mucosa, leading to sexually transmitted diseases. Genital herpes is one of the most prevalent sexually transmitted diseases worldwide, especially in immuno-compromised population.1 And HSV-2 infection increases the risk of HIV infection.2, 3 Moreover, HSV-2 transmission can occur via sexual and perinatal route during asymptomatic viral shedding.3 There are several drugs licensed for the treatment of HSV infections and most of them target viral DNA polymerase. Because of the rapid emergence of drug-resistant virus strains, the development of effective therapies for HSV-caused diseases becomes more and more important, especially the development of novel therapeutic agents with different mechanisms of action.
Geldanamycin (GA) is an antibiotic with its primary target on the ADP/ATP-binding site of heat shock protein 90 (Hsp90). GA binding blocks the activity of Hsp90 chaperone function and results in rapid degradation of Hsp90-associated client proteins via the ubiquitin-proteasome pathway. These client proteins include RAF-1, ERRB-2, CDK4 and AKT/PKB, p53, pRb and steroid hormone receptors.4, 5 As a specific inhibitor of Hsp90, GA and its derivatives display antitumor activities in a multitude of animal models.6, 7 17-AAG, a derivative of GA, is now in clinical trial for the treatment of various cancers, which include multiple myeloma (phase III), breast, renal, thyroid, ovarian, pancreas (phase II), pediatric solid tumors (phase I).8, 9
In the field of virology, Hsp90 is important for several virus replications, including HBV, HCV, HCMV, HSV, VSV, vaccinia virus, coxsackie virus, rhinovirus and Ebola virus.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 It has been shown that Hsp90 specific inhibitor GA blocks the replication of the viruses mentioned above in cell culture systems. We also confirmed that GA significantly decreased the mortality and increased the mean survival day of mice in HSV-1 mouse encephalitis model.20
In this study, anti-HSV-2 activities of GA were evaluated both in cell culture and in a genital model of mice infected with HSV-2.
Materials and methods
Animals
Female Kunming mice (5–6 weeks in age and 13–15 g in weight) were obtained from the Center of Experimental Animals, Chinese Academy of Medical Sciences in Beijing, China. Mice were maintained at the animal facility of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, under permit of the Ethical Committee for Animal Experiments of the Institute of Medicinal Biotechnology. Mice were distributed randomly into groups (6 or 10 mice per group) with wood chip bedding and provided with chow pellets and tap water ad libitum. The mice were housed at 21–24 °C and 40–60% humidity with a 12-h light–dark cycle.
Cells and viruses
African green monkey kidney (Vero) cells and HSV-2 (333 strain) were obtained from the Institute of Virology, Chinese Academy of Medical Sciences in Beijing, China. Cells were grown in Eagle’s Minimum Essential Medium supplemented with 10% heat inactivated fetal bovine serum, supplemented with penicillin at 100 U ml−1, streptomycin at 100 μg ml−1, and 2 mM L-glutamine. HSV-2 was propagated in Vero cells. After three cycles of freeze–thawing and a brief centrifugation, the supernatant was aliquoted and kept at −80 °C until use, while virus titer of the supernatant was determined by plaque formation assay.
Compounds
GA was purchased from Sigma Chemicals (St Louis, MO, USA). It was dissolved in DMSO at 10 or 100 mM as a stock solution and further diluted in culture medium or 0.1% Tween-80 before use. Acyclovir (ACV) was kindly provided by the Department of Chemical Drugs, the Chinese State Drug Administration. It was dissolved in sterile water as a stock solution and further diluted in culture medium or saline before use.
Cytotoxicity determination in cell culture
Vero cells in exponential growth were seeded into 96-well microplates (Falcon, Oxnard, CA, USA) at a density of 2.5 × 104 cells per well, followed by addition of GA at final concentrations between 0–1000 μM. After incubation at 37 °C for 72 h, cell viability was assessed by MTT assay (3-(4,5-dimethylthiazol-2,5-diphenyl tetrazolium bromide.18 Cytotoxicity was determined in duplicate, and each experiment was repeated three times under identical conditions. CC50 was defined as the concentration that inhibits 50% cellular growth in comparison with untreated controls and calculated by Reed and Muench method.
Anti-HSV-2 activity in vitro
Confluent Vero cells grown in six-well plates were infected with 100 PFU HSV-2 (strain 333) per well. After 1-h adsorption at 37 °C, cell monolayers were washed with phosphate-buffered saline and maintained with culture medium (MEM containing 1% methylcellulose) with or without various concentrations of GA. After 3-day incubation at 37 °C in 5% CO2, the plates were fixed with 10% buffered Formalin aqueous, and stained with 0.5% crystal violet in 20% methanol. Plaques were counted. The IC50 was determined by Reed & Muench method.
Virus titration by the cytopathic effect method for virus shedding experiment
Confluent cell cultures grown in 96-well microplates were infected in duplicate with consecutive 10-fold dilutions of each viral sample collected from each mouse of different groups. After 1-h adsorption at 37 °C, the monolayers were washed by phosphate-buffered saline and incubated at 37 °C in the maintenance medium (MEM plus 2% fetal bovine serum). Viral cytopathic effect was observed and the TCID50 was determined by the Reed & Muench method.
Inoculation of kunming mice
We used a mouse vaginal model of HSV-2 infection with modification to study in vivo anti-HSV-2 efficacy of GA.21 Briefly, Mice vaginas were extensively cleaned by sterile cotton swab. A 20-μl solution containing HSV-2 (2 × 105 PFU ml−1) prepared in MEM was inoculated intravaginally. The virus doses used in all infected experiments resulted in a high rate of mortality (>80%).
Antiviral effect in vivo
In vagina model (10 mice per group), the mice were treated intravaginally with three different concentrations of GA suspension (5.72, 2.86 and 1.43 mg kg−1) 1 h post infection, three times a day for 7 days. The same procedure was applied to ACV group (2.86 mg kg−1) and the infected control group (vehicle). Animals were monitored for morbidity and mortality for 14 days.
Virus shedding experiment
Mice were randomly grouped (six in each group), color labeled and inoculated intravaginally with HSV-2. GA treatment was done in the same way as described above, except the duration of treatment was 4 days. Samples of vaginal secretions from all mice in each group were collected individually at 96 h post infection. For sampling, the cotton sterile swab wetted by MEM were put into the vaginas for 5 s and very gently swabbed. Then the cotton swabs were put into a test tube with 1-ml MEM and antibiotics (penicillin 100 U ml−1, streptomycin 100 μg ml−1) as soon as possible. Samples were frozen at −70 °C until titration was performed by cytopathic effect assay.
Drug toxicity in vivo
Uninfected mice were grouped and treated with the same doses as the infected mice groups. Mice were weighed individually on days 0, 7 and 14.
Statistical analysis
Statistical significance was estimated according to χ2 analysis for mortality data, Kaplan–Meier analysis for mean survival days and t-tests analysis for body weight by using SPSS software (SPSS Software). A P-value of 0.05 or less was considered as significant.
Results
In vitro anti-HSV-2 activities of GA
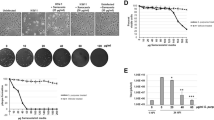
We used plaque reduction assay to study anti-HSV-2 activities of GA. GA significantly inhibited HSV-2 replication in Vero cells in a dose-dependent manner (Figure 1). IC50 values of GA were 0.171±0.001 μM. The cytotoxic effect of GA on Vero cells was measured with a conventional MTT method, and the CC50 was 350 μM. The therapeutic index of GA for HSV-1 infection in Vero cells was 2047, better than that known for the HSV drug ACV (Tab1e 1).
Geldanamycin (GA) inhibited Herpes simplex virus type 2 (HSV-2) replication in Vero cells in a dose-dependent manner. HSV-2 infected Vero cells were incubated in the absence or presence of GA at final concentration in the 0–0.5 μM range (0, 0.0625, 0.125, 0.25, 0.5 μM). Viral-reduced plaque assay was determined on 72 h post infection. A full color version of this figure is available at The Journal of Antibiotics journal online.
In vivo anti-HSV-2 activities of GA
We evaluated the in vivo anti-HSV-2 activity of GA in the mouse HSV-2 vagina model.
In preliminary experiments, Kunming mice were inoculated with variable doses of HSV-2 to find an adequate virus dose that would cause death of majority of infected mice. In formal experiments, virus doses used resulted in a high rate of mortality (>80%).
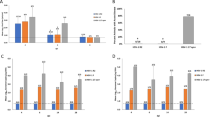
In mouse HSV-2 vaginal model, symptoms of illness that were observed in mice after vaginal HSV-2 infection include: puff fur, arched backs, feeble gait, hind limb paralysis, wet fur that was stained by feces in the anus vaginal region, swollen red vulva, hair loss and skin lesions in the anus vaginal region and on the hind limbs, and death. Disease signs evolved from inflammation and redness, to hair loss, ulcerous lesions in the genital region and finally flaccid paralysis. In this study, HSV-2 infected mice died around 5 days post infection if no treatment was involved. Administration of GA suspension to vagina after HSV-2 infection (1.43, 2.86 and 5.72 mg kg−1, t.i.d. for 7 days) protected the infected mice from death, and increased the average survival days in a dose-dependent manner. The differences were statistically significant when compared with untreated infected controls (Table 2).
As shown in Figure 2, GA also significantly reduced the shedding of HSV-2 from mice vagina. Samples of vaginal secretions from all mice in each group were collected individually at 96 h post infection. HSV-2 titers were determined by a cytopathic effect assay. GA 5.72, 2.86 and 1.43 mg kg−1 treatment reduced HSV-2 shedding by 794, 126 and 126 times, as compared with the infected control group, respectively. ACV 2.86 mg kg−1 treatment reduced HSV-2 shedding by 31.6 times, as compared with the infected control group.
Mice were randomly grouped and inoculated intravaginally with Herpes simplex virus type 2 (HSV-2). Geldanamycin (GA) treatment was done in the same way from 1 h post infection, three times a day for 4 days. Samples of vaginal secretions from all mice in each group were collected individually at 96 h post infection. Samples were frozen at −70 °C until titration performed by cytopathic effect assay.
In summary, GA efficiently inhibits HSV-2 replication in vivo and shows therapeutic effects better than that of ACV.
Mouse growth impact by GA in mouse vaginal model
Uninfected mice were grouped and treated with the same doses as the infected mice groups. Mice were weighed individually on days 0, 7 and 14. As shown in Table 3, All GA vaginal administration groups had no effects on mice growth by weight measurement.
Discussion
Hsp90 has crucial roles in the maintenance of the conformation, stability, activity and cellular localization of several key proteins that are involved in cell signaling, proliferation and survival. Hsp90 is a novel target for cancer therapy.22, 23 Hsp90 is important for several virus replications and Hsp90 specific inhibitor GA blocks the replication of the viruses mentioned above in cell culture systems.11, 12, 13, 14, 15, 16, 17, 18, 19
Most antiviral drugs in clinic use target a specific viral protein ensuring their specificity and limited toxicity. Though great progresses have been made through this approach, it is obvious that antiviral drugs targeting viral proteins have a relatively narrow antiviral spectrum and associate with the emergence of drug-resistant viral strains. Therefore, it is worthwhile to develop new antivirals with different mechanisms of actions. Targeting a cellular protein related to virus replication is a potential alternative approach for antiviral drug development. It will generate inhibitors with broad antiviral spectrum and less likelihood of emergence of drug resistance.
We reported GA, a ligand of heat shock protein 90, effectively inhibited HSV-1 in vitro. And the mechanism of GA against HSV-1 were explored.18 The antiviral mechanism of GA against HSV-1 appears to be associated with Hsp90 inactivation and cell cycle restoration, which indicates that GA exhibits broad-spectrum antiviral activity. In fact, GA exhibited broad-spectrum antiviral activity against HSV-2, VSV, Coxsackie virus B3, HIV-1, SARS coronavirus in vitro.18 And other paper reported GA effectively inhibited replication of HCMV, HPIV-2, HPIV-3, poliovirus, rhinovirus.15, 16
GA exhibited antiviral activity in vitro was reported in many papers.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Relevant reports about antiviral activity of GA in vivo are relatively rare. We confirmed GA effectively inhibited HSV-1 in vivo in our previous work.20 GA were administrated by intraperitoneal injection and subcutaneous injection and exhibited inhibitory effect against HSV-1 in mouse encephalitis model.20
It is difficult to develop systemic administration of GA because of low water solubility and high hepatotoxicity.23 So we did lot of work for modification of GA and got some GA derivatives. We have discussed the structure–activity relationship of GA derivatives in HCV replicon system19 and HSV-1, HSV-2, HCMV, HBV, Coxsackie virus B3, Coxsackie virus B6.24 Overall, the cytotoxicity of most of the tested derivatives was decreased as compared with the lead compound GA. But the antiviral activity against HSV-2 of most of the tested derivatives19 except one compound was also reduced compared with GA (see Supplementary data).
Topical treatment is very important for cutaneous HSV infection. Topical treatment can reduce systemic exposure to drug side effects and reach high tissue drug level. So our study in this paper focuses on GA topical treatment HSV-2 infection in mouse vaginal model. In mouse vagina model, administration of GA suspension to vagina after virus infection protected the infected mice from death and increased the average survival days and significantly reduced virus shedding from mouse vagina in a dose-dependent manner. GA effectively inhibited HSV-2 replication in mouse vaginal model by topical treatment. At the same time, GA vaginal administration groups had no effects on mice growth by weight measurement. The high-dose group of GA was up to 5.72 mg kg−1.
Our findings that GA effectively inhibited HSV-1 and HSV-2 replication both in vitro and in vivo further consolidate our opinion that Hsp90 is a potential novel target for antiviral research. Although cellular protein inhibitors might associate with apparent toxicities, they might be attenuated by administrating properly or reducing dosage through a combination with a viral protein-targeting drug. More importantly, target on Hsp90 may provide an antiviral approach refractory to development of drug resistance.16
GA is not suitable for systemic administration because of its toxicity. We consider that GA is a candidate of topical usage for the treatment of HSV infections.
References
Roizman, B., Knipe, D. M. & Whitley, R. J. Herpes Simplex Virus in Fields Virology, 5th ed. (eds Knipe D. M., Howley P. M.) 2552–2558 (Lippincott, Williams & Wilkins: Philadelphia, PA, Baltimore, MD, USA, 2006).
Martinelli, E. et al. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog. 7, e1002109 (2011).
Schiffer, J. T. & Corey, L. New concepts in understanding genital herpes. Curr. Infect. Dis. Rep. 11, 457–464 (2009).
Blagosklonny, M. V. Hsp-90-associated oncoproteins: multiple targets for geldanamycin and its analogs. Leukemia 16, 455–462 (2002).
Richter, K. & Buckner, J. Hsp90: chaperoning signal transduction. J. Cell Physiol. 188, 281–290 (2001).
Ochel, H. J., Eichhorn, K. & Gademann, G. Geldanamycin: the prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones 6, 105–112 (2001).
Miyata, Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 11, 1131–1138 (2005).
Biamonte, M. A. et al. Heat shock protein 90: inhibitors in clinical trials. J. Med. Chem. 53, 3–17 (2010).
Pacey, S., Banerji, U., Judson, I. & Workman, P. Hsp90 inhibitors in the clinic. Handb. Exp. Pharmacol. 172, 331–358 (2006).
Hu, J. & Seeger, C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl Acad. Sci. USA 93, 1060–1064 (1996).
Hu, J., Toft, D. O. & Seeger, C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO. J. 16, 59–68 (1997).
Waxman, L., Whitney, M., Pollok, B. A., Kuo, L. C. & Darke, P. L. Host cell factor requirement for hepatitis C virus enzyme maturation. Proc. Natl Acad. Sci. USA 98, 13931–13935 (2001).
Burch, A. D. & Weller, S. K. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J. Virol 79, 10740–10749 (2005).
Basha, W. et al. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibit gene expression and replication of human cytomegalovirus. Antivir. Chem. Chemother. 16, 135–146 (2005).
Connor, J. H., McKenzie, M. O., Parks, G. D. & Lyles, D. S. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology 362, 109–119 (2007).
Geller, R., Vignuzzi, M., Andino, R. & Frydman, J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Gen. Dev. 21, 195–205 (2007).
Smith, D. R. et al. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral. Res. 87, 187–194 (2010).
Li, Y. H., Tao, P. Z., Liu, Y. Z. & Jiang, J. D. Geldanamycin, a ligand of heat shock protein 90, inhibits the replication herpes simplex virus type 1 in vitro. Antimicrob. Agents Chemother. 48, 867–872 (2004).
Shan, G. Z. et al. A novel class of geldanamycin derivatives as HCV replication inhibitors targeting on Hsp90: synthesis, structure-activity relationships and anti-HCV activity in GS4.3 replicon cells. J. Antibiot. 64, 177–182 (2011).
Li, Y. H. et al. Inhibition of Herpes Simplex Virus Type 1 Infection In Vitro and In Vivo by Geldanamycin. Chin. J. Antibiot. 29, 311–315 (2004).
Kokuba, H., Aurelian, L. & Neurath, A. R. 3-Hydroxyphthaloyl beta-lactoglobulin. IV Antiviral activity in the mouse model of genital herpesvirus infection. Antivir. Chem. Chemother. 9, 353–357 (1998).
Goetz, M. P., Toft, D. O., Ames, M. M. & Erlichman, C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann. Oncol. 14, 1169–1176 (2003).
Gorska, M. et al. Geldanamycin and its derivatives as Hsp90 inhibitors. Front Biosci. 17, 2269–2277 (2012).
Li, Y. P. et al. Synthesis and biological evaluation of heat-shock protein 90 inhibitors: geldanamycin derivatives with broad antiviral activities. Antivir. Chem. Chemother. 20, 259–268 (2010).
Acknowledgements
The work was supported by the National S&T Major Special Project on Major New Drug Innovation (2012ZX09301002-001), PR China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, YH., Lu, QN., Wang, HQ. et al. Geldanamycin, a ligand of heat shock protein 90, inhibits herpes simplex virus type 2 replication both in vitro and in vivo. J Antibiot 65, 509–512 (2012). https://doi.org/10.1038/ja.2012.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2012.67