Abstract
Tobramycin is an aminoglycoside antibiotic that loses a significant amount of activity in the presence of Zosyn at pH 6. As part of our investigation into ways to improve the compatibility of tobramycin with Zosyn (which contains piperacillin and tazobactam in an 8:1 ratio buffered at pH 6 by sodium citrate) by lowering the pH, we identified the reaction product of tobramycin and piperacillin at pH 6.0 and the order of the pKa values of tobramycin. The structure of the main reaction product of tobramycin and piperacillin at pH 6.0 was determined by 2D NMR to be the product of 3″-NH2 reacting with the β-lactam of piperacillin. The order of the pKa values of the nitrogens of tobramycin was determined by 1H and 15N NMR titrations to be 6′-NH2>2′-NH2>1-NH2≈3″-NH2>3-NH2. At pH 4.0, the reaction between tobramycin and Zosyn was almost negligible for a period of up to 2 h. The pH can be lowered by adding an acid such as HCl or citric acid to Zosyn to make a pH 4.0 buffer.
Similar content being viewed by others
Introduction
Tobramycin (Figure 1) is an aminoglycoside antibiotic produced by Streptomyces tenebrarius1 used to treat infections caused by susceptible Gram-negative microorganisms.2 It is often used in combination with penicillins, such as piperacillin (Figure 2), to treat severe infections caused by Gram-negative bacteria.3 It is well known that tobramycin reacts with β-lactams, including piperacillin, causing a significant loss of aminoglycoside activity.4 The inactivation mechanism is thought to involve nucleophilic attack and ring opening of the penicillin β-lactam ring by an amino group of the aminoglycoside.5, 6 The rate of aminoglycoside inactivation is dependent on temperature, time, concentration of the β-lactam and composition of the medium.7, 8, 9 Another important factor to consider is the pH of the solution. As the degree of protonation of an amino group increases, it should become less nucleophilic. The amino groups of tobramycin have different pKa values,10, 11 hence, it is logical to assume the reaction would be affected by pH of the solution. Therefore, knowledge of the protonation constants of the amino groups and their order is important in understanding the reactivity of tobramycin with piperacillin at different pHs.
Zosyn is an intravenously administered antibiotic, which contains piperacillin and tazobactam (a β-lactamase inhibitor) in an 8:1 ratio. It also contains EDTA and sodium citrate, which is used to buffer the solution at around pH 6.12 It has been approved for Y-site co-administration with the aminoglycosides amikacin and gentamicin.13 It has not been approved for co-administration with tobramycin, which is not as stable as amikacin and gentamicin in the presence of β-lactam antibiotics.9
As part of our investigation into ways to improve the compatibility of tobramycin with Zosyn by lowering the pH, we identified the main reaction products of tobramycin and piperacillin at pH 6.0 and the order of the pKa values of the tobramycin amino groups. Although some effort has been made to identify the reaction products of aminoglycosides with carbenicillin,6 the exact product formed when tobramycin is inactivated by piperacillin has not been reported. Here we report the structures of the main reaction products of tobramycin and piperacillin at pH 6.0.
Results and discussion
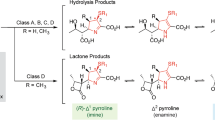
Piperacillin and tobramycin were reacted at pH 6.0 at room temperature (∼23 °C) in a 1:1 ratio for 2 days. After analyzing the mixture by HPLC and LC-MS, it was found that only two main reaction products were formed, in a 4:1 ratio. They were isolated by preparative HPLC and their structures (Figure 3) were determined by 2D NMR (see Table 1 for assignments). The ESI mass spectra recorded in positive-ion mode showed a molecular ion peak at m/z 985 [M+H]+, indicating a molecular weight of 984 for both reaction products. The molecular formula of both compounds was determined to be C41H66N10O16S, based on the HRESIMS spectral data (calcd: 493.2184 for [M+2H]2+, found: 493.2187 for 1 and 493.2183 for 2). The major reaction product (1) was found to result from the reaction of the 3″-NH2 amino group with β-lactam, retaining the stereochemistry of the piperacillin moiety. The other product (2) is simply an epimer of the first at the P2 position. Evidence for the formation of an amide between 3″-NH2 and the β-lactam carbonyl linking the piperacillin and tobramycin moieties comes from the HMBC spectra. The HMBC spectra for both 1 and 2 show long range 1H-13C correlations from both H3″ and 3″-NH to the P7 carbonyl carbon of the piperacillin moiety. P6H also shows a long-range 1H-13C correlation to P7 in the spectra of both compounds, confirming the assignment of this carbonyl (Figure 4). The stereochemistries of the two epimers were determined from the ROESY spectra. In the ROESY spectrum of 2, there is a correlation observed between P2H and P5H, indicating that they are both on the same side of the five-member ring (Figure 4). This correlation is absent in the ROESY spectrum of 1. Further evidence for the stereochemistry of 1 comes from the fact that in the HMBC spectrum P2H shows a strong three-bond 1H-13C correlation to P26, but a much weaker one P27, indicating that P2H is anti-coplanar with P26 (Figure 4). In the ROESY spectrum, there is a correlation observed between P26H and P5H, indicating that they are both on the same side of the five-member ring, whereas P2H is on the opposite side (Figure 4).
Our results differ from a previous study on the reaction of aminoglycosides with carbenicillin.6 After reacting tobramycin with about a threefold excess of carbenicillin at room temperature for 7 days, the site of reaction was determined by comparing acid hydrolysis products of the starting tobramycin and the reaction product. It was reported that, although the acylation of tobramycin was not specific, most of the reaction occurred at 3-NH2 with a smaller amount occurring at 6′-NH2 or 2′-NH2. However, the structure of the reaction product was not directly elucidated and was inferred based on hydrolysis and 13C chemical shifts of the carbenicillin derivative at pH 4 compared with the shifts of amikacin and isoamikacin at neutral pH. Also, the exact pH of the reaction mixture used in the study was not stated. It is possible that the pH was higher leading to a different reaction than our study.
As the structures of the major reaction products of tobramycin and piperacillin indicate that N3″ is the most reactive nitrogen at pH 6.0, we took a closer look at the protonation constants of tobramycin to examine their role in determining the reaction products. The pKa values of tobramycin have been measured by both potentiometric10 and 15N NMR11 titrations, with only one pKa <7, but the order of the protonation constants has not been clearly established. Therefore, we performed NMR titrations using the 1H and 15N signals of tobramycin (Figure 5) to unambiguously determine the order of pKa values of tobramycin. The pKa values were determined from the inflection points on the titration curves. The assignments of the 1H signals at low pH were made by direct comparison with the literature assignments at low pH,14 and were confirmed from HSQC spectra by comparing the 13C assignments to the literature assignments made at low pH. HSQC spectra were then used to follow the shifts as the pH changed. A comparison of the assignments at high pH to the literature assignments14, 15 was used to confirm that for each curve, the same 1H signal was followed throughout the titration. The 15N resonances were assigned and their shifts determined from 1H-15N HMBC spectra. The values determined by the 1H NMR and 15N NMR titrations are in close agreement with each other. Our values agree with those found by the previous 15N NMR titration11 (performed in H2O), after correcting for the deuterium isotope effect16 for our titrations performed in D2O (Table 2). However, only tentative assignments were made for N1 and N3 in that study and we found the assignments of N1 and N3 to be interchanged. Therefore, the order of the protonation constants is: 6′-NH2>2′-NH2>1-NH2≈3″-NH2>3-NH2. The pKa values for 1-NH2 and 3″-NH2 are very similar, whereas the pKa for 3-NH2 is significantly lower and the only one <7.
Based solely on the pKa values of tobramycin, one might expect the most likely product of piperacillin and tobramycin at pH 6.0 to be an amide formed by 3-NH2 opening the β-lactam ring; this is clearly not the case. Hydrogen bonding of 3″-NH2 to the flanking hydroxyl groups may make it less protonated and more nucleophilic at pH 6.0 than its pKa would suggest. The apparent lower pKa of 3-NH2 is probably due to the fact that protonation of 3-NH2 is more difficult after 1-NH2 is protonated because of electrostatic interactions, and its intrinsic pKa would be significantly higher.17 This along with other factors, such as steric constraints, affects its reactivity leaving 3″-NH2 as the most reactive amino group at pH 6.0. Once the pH of the solution is considerably below the pKa of 3″-NH2, the reaction is much slower. After 4 days at room temperature, there is very little reaction of piperacillin and tobramycin detected at pH 4.0.
Using a variation of the NMR method developed for determining the compatibility of gentamicin with Zosyn,13 we confirmed that tobramycin is not as stable as gentamicin in the presence of Zosyn at pH 6.0 (Table 3). To test the effect of pH on the stability of tobramycin with Zosyn, a series of experiments were performed using piperacillin, the main component of Zosyn, and tobramycin in concentrations that are higher (120 and 10 mg ml−1, respectively), but within the same range as the ratios used clinically. At pH 6.0, it was found that 40% of the initial amount of tobramycin was lost in 60 min. As the pH decreases below 5.0 the reaction slows significantly; at pH 4.6 and lower nearly 97% of the tobramycin remained after 60 min. The best stability was observed at pH 4.0. Next, we tested whether tobramycin is stable with Zosyn itself at clinically relevant concentrations. As the data at pH 6 show the worst stability at the high Zosyn (48.72 mg ml−1)—low tobramycin (0.35 mg ml−1) concentrations, we used that combination to test the stability at pH 4.0. There is no significant decrease in tobramycin in the presence of Zosyn at pH 4.0 over 60 min and even at 120 min. This finding was confirmed by LC-MS.
In our studies, we lowered the pH using HCl (DCl) or citric acid. As the use of HCl could be hazardous in a non-laboratory setting, using citric acid makes sense for possible clinical use. The formulation of Zosyn already contains a citrate buffer and adding citric acid would make a lower pH buffer that would keep the pH constant, even with the use of different diluents normally used in a hospital setting. Zosyn contains 4.4% sodium citrate to buffer it at pH 6.0.12 Using the pK values of citric acid, the amount of citric acid needed to lower the pH to 4.0 was calculated to be 0.048 times the amount of Zosyn added (4.4% × 1.1 for citric acid/citrate ratio). This was verified experimentally and was used in the LC-MS experiment above. Although more work needs to be carried out to validate methods to prove that tobramycin and piperacillin remain stable at pH 4.0 for at least an hour, this work could eventually lead to a means for Y-site co-administration of tobramycin and Zosyn in patients.
Experimental Procedure
Materials
Piperacillin and tobramycin were purchased from Sigma (St Louis, MO, USA). Zosyn was obtained from Wyeth (Pearl River, NY, USA). D2O and DMSO-d6 were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). NaOD, DCl and citric acid were purchased from Aldrich (Milwaukee, WI, USA).
General
All NMR spectra were acquired at 25 °C on either a Bruker DRX-400 NMR spectrometer (Bruker, Billerica, MA, USA) equipped with a QNP probe or a Bruker DRX-500 NMR spectrometer equipped with a TXI probe. Samples for the NMR titrations were prepared in D2O with sample concentrations of 0.1–0.2 M with acetonitrile added as a reference. The pH meter was calibrated with standard aqueous buffers at pHs 4.0 and 7.0. The apparent pD (pD*) was adjusted using either DCl or NaOD. Actual pD values were determined using the equation,18 pD=pD*+0.40, and were used in Figure 5. The 15N shifts were determined from 1H-15N HMBC spectra acquired on the Bruker DRX-500 NMR spectrometer and were referenced relative to liquid NH3. Samples of the reaction products of piperacillin and tobramycin were prepared in DMSO-d6 and the following spectra were acquired for each sample: proton, 13C, COSY, TOCSY, ROESY, HSQC and HMBC. 1H chemical shifts were measured relative to TMS, and 13C chemical shifts were measured with relative to the DMSO-d6 signal at δ 39.5.
Preparation and isolation of the reaction products of piperacillin and tobramycin
Piperacillin sodium (54 mg) and tobramycin sulfate (47 mg) were dissolved in water (1.5 ml), and allowed to stand for 2 days at room temperature (∼23 °C). The reaction mixture was then chromatographed using an Agilent 1100 system (Agilent, Santa Clara, CA, USA) with a semi-preparative reversed-phase column (Varian Inersil 5u ODS-3, 250 × 10 mm) equipped with a DYNAMAX fraction collector (Rainin, Oakland, CA, USA) (Model FC-1). The column was washed with a gradient of 10–22% acetonitrile (A) in 0.05% trifluoroacetic acid buffer (B) over 25 min at a flow rate of 4 ml min−1 with UV detection at 210 nm. A total of 10 injections was performed, and peaks corresponding to 2 (20.0 min) and 1 (21.6 min) were repeatedly collected. The collected fractions were then concentrated to dryness by Speed-Vac to yield 2 (2 mg) and 1 (8 mg).
Monitoring the stability of tobramycin in the presence of piperacillin or Zosyn by NMR
Using a method similar to that described by Desai et al.,13 the stability of tobramycin as a function of time in the presence of piperacillin or Zosyn was monitored by the comparative integration of the internal standard (hydroquinone monoethyl ether) signals between δH 7.00 and δH 6.60, which was normalized to 1.00, to the integral of the H1′ proton of tobramycin between δH 5.85 and δH 5.65 (the chemical shift of this proton changes with pH, but is within this range for the pH values studied).
Monitoring the stability of tobramycin in the presence of zosyn at pH 4.0 by LC-MS
A solution of 243.6 mg Zosyn, 1.8 mg tobramycin and 11.8 mg citric acid in 5 ml H2O was used for the MS experiments. The addition of citric acid lowered the pH to 4.0. An Agilent 1100 HPLC system coupled with an Applied Biosystems-PE SCIEX QSTAR PULSAR i quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with an electrospray ionization ion source operated in the positive ionization mode was used for the LC-MS experiments. The normalized peak intensity of the [M+Na]+ ion (m/z 490) of tobramycin was monitored as a function of time. The citric acid peak ([M+H]+ ion of m/z 193) was used for normalization.
References
Higgins, C. E. & Kastner, R. E. Nebramycin, a new broad-spectrum antiobiotic complex. II. Description of Streptomyces tenebrarius. Antimicrob. Agents Chemother. 7, 324–331 (1967).
Siegenthaler, W. E., Bonetti, A. & Luthy, R. Aminoglycoside antibiotics in infectious diseases: an overview. Am. J. Med. 80 (6, Suppl 2), 2–14 (1986).
Lynch, J. P. Hospital-acquired pneumonia: risk factors, microbiology, and treatment. Chest 119, 373S–384S (2001).
Glew, R. H. & Pavuk, R. A. Stability of gentamicin, tobramycin, and amikacin in combination with four β-lactam antibiotics. Antimicrob. Agents Chemother. 24, 474–477 (1983).
Page, M. I. The mechanisms of reactions of β-lactam antibiotics. Acc. Chem. Res. 17, 144–151 (1984).
Iyengar, B. S., Kumar, V., Wunz, T. P. & Remers, W. A. Aminoglycoside antibiotics. 6. Chemical reactions of aminoglycosides with disodium carbenicillin. J. Med. Chem. 29, 611–614 (1986).
Riff, L. J. & Jackson, G. G. Laboratory and clinical conditions for gentamicin inactivation by carbenicillin. Arch. Intern. Med. 130, 887–891 (1972).
Pickering, L. K. & Gearhart, P. Effect of time and concentration upon interaction between gentamicin, tobramycin, netilmicin, or amikacin and carbenicillin or ticarcillin. Antimicrob. Agents Chemother. 15, 592–596 (1979).
Pickering, L. K. & Rutherford, I. Effect of concentration and time upon inactivation of tobramycin, gentamicin, netilmicin and amikacin by azlocillin, carbenicillin, mecillinam, mezlocillin and piperacillin. J. Pharmacol. Exp. Ther. 217, 345–349 (1981).
Jeżowska-Bojczuk, M., Karaczyn, A. & Kozłowski, H. Copper (II) binding to tobramycin: potentiometric and spectroscopic studies. Carbohyr. Res. 313, 265–269 (1998).
Dorman, D. E., Paschal, J. W. & Merkel, K. E. 15N nuclear magnetic resonance spectroscopy. The nebramycin aminoglycosides. J. Am. Chem. Soc. 98, 6885–6888 (1976).
Prescribing Information for Zosyn® in Galaxy® Containers (2009). Available at http://labeling.pfizer.com/showlabeling.aspx?id=436.
Desai, N. R., Shah, S. M., Cohen, J., McLaughlin, M. & Dalal, H. R. Zosyn® (piperacillin/tazobactam) reformulation: expanded compatibility and coadministration with lactated Ringer's solutions and selected aminoglycosides. Ther. Clin. Risk Manag. 4, 303–314 (2008).
Szilágyi, L. Assignments of the 1H- and 13C-N.M.R. spectra of tobramycin at low and high pH. Carbohydr. Res. 170, 1–17 (1987).
Eneva, G. I., Spassov, S. L., Haimova, M. A. & Sandström, J. Complete 1H and 13C NMR assignments for apramycin, sisomicin and some N- and N,O-polyacetylated aminoglycosides. Magn. Reson. Chem. 30, 841–846 (1992).
Delgado, R. et al. Dissociation constants of Brønsted acids in D2O and H2O: studies on polyaza and polyoxa-polyaza macrocycles and a general correlation. Anal Chim Acta 245, 271–282 (1991).
Onufriev, A., Case, D. A. & Ullmann, G. M. A novel view of pH titration in biomolecules. Biochemistry 40, 3413–3419 (2001).
Glasoe, P. K. & Long, F. A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 64, 188–190 (1960).
Acknowledgements
We thank Narendra Desai and Hema Dalal for supplying the Zosyn samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wyeth was acquired by Pfizer on 16 October 2009.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Pagano, T., Gong, Y., Kong, F. et al. Structural characterization of the tobramycin–piperacillin reaction product formed at pH 6.0. J Antibiot 64, 673–677 (2011). https://doi.org/10.1038/ja.2011.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2011.72