Abstract
A novel metabolite, LL-Z1272α epoxide, structurally related to ascochlorin, was isolated from the cultured mycelium of Ascochyta viciae J-29, a mutant derived from A. viciae Libert. The structure was elucidated on the basis of spectroscopic data. The epoxide is proposed to be enzymatically formed from LL-Z1272α and is a precursor of ascochlorin, an antiviral and antitumor antibiotic. The conversion of the epoxide to ascochlorin by cyclization of its farnesyl chain to a cyclohexanone ring is similar to that of squalene 2, 3-oxide to sterols. Unlike ascochlorin, the new metabolite had no growth inhibitory activity against Candida albicans in the paper-disc agar diffusion assay.
Similar content being viewed by others
Introduction
Ascochlorin was originally isolated as an antiviral agent from the filter cake of the fermented broth of Ascochyta viciae Libert1 (Figure 1), and ascofuranone was similarly isolated as an antiviral compound from the same strain.2 Subsequently, many structurally related compounds have been isolated from various fungi such as an unclassified Fusarium species LL-Z1272,3 Cylindrocladium sp.,4 Cylindrocladium ilicicola MFC-870,5 Nectria coccinea,6 Colletotrichum nicotianae,7 Acremonium luzulae,8 Cephalosporium diospyri,9 Verticillium sp. FO-2787,10 Cylindrocarpon lucidum,11 Nigrosabulum globosum12 and the insect pathogenic fungus Verticillium hemipterigenum BCC 2370.13
Ascochlorin and its derivatives exhibit a large variety of physiological activities, including hypolipidemic activity,14 suppression of hypertension,15 amelioration of type I and II diabetes,16 antitumor activity17 and immunomodulation.18
Although ascochlorin and structurally related compounds have been isolated from diverse fungi and have been reported to have wide biological activities, their biosynthetic pathway has been uncertain. It has been proposed that the farnesyl chain of LL-Z1272α (Figure 1) is epoxidized, cyclized to a cyclohexanone ring and converted to ascochlorin just as in the case of the enzymatic conversion of squalene 2, 3-oxide to lanosterol and cholesterol.3 However, this epoxide, the expected intermediate to which the farnesyl chain with a terminal double bond is converted, has not been isolated hitherto.
By screening metabolites produced by more than 2000 mutants of A. viciae, we found a new compound related to ascochlorin. The structure was elucidated to be LL-Z1272α epoxide on the basis of spectroscopic data. In this report, we describe the isolation and structure of this epoxide (Figure 1) and discuss the biosynthesis of ascochlorin.
Results
Physico-chemical properties of LL-Z1272α epoxide
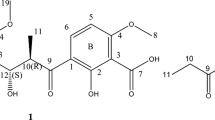
LL-Z1272α epoxides are crystals with a pale yellow color; Mp: 38–39 °C; [α]25.4D=+6.6 (c 0.5, CHCl3); high resolution fast atom bombardment mass spectrum (HRFAB-MS) m/z 407.1941 [M+H]+ (calculated for C23H32ClO4, 407.1989); UV (MeOH) λmax (ɛ) 227 (17 000) and 293 (12 900) nm; IR (KBr) νmax 3384, 2984–2834, 1617, 1279, 1243 cm−1. The 1H-NMR and 13C-NMR assignments of LL-Z1272α epoxide are listed in Table 1.
Structure elucidation
The LL-Z1272α epoxide was obtained as crystals with a pale yellow color. Its formula was determined to be C23H31ClO4 on the basis of HRFAB-MS at m/z 407.1941 [M+H]+ (calculated for C23H32ClO4, 407.1989). IR absorptions at 3384 (OH), 2984–2834 (methyl, methylene and methine), 1617 (CO), 1279 (CO) and 1243 (CO) cm−1 were observed. UV spectral absorptions at λmax (ɛ) 227 (17 000) and 293 (12 900) nm were obtained. The 13C-NMR spectrum (CDCl3) showed 23 resolved peaks, which were classified into 5 sp3 methyls (δc 14.5, 16.2, 16.0, 23.4 and 26.5), 5 sp3 methylenes (δc 22.1, 39.6, 26.2, 36.8 and 36.8), 1 sp3 methine (δc 78.3), 2 sp2 methines (δc 121.3 and 125.0), 1 sp3 oxygenated quarternary carbon atom (δc 73.1), 8 sp2 quarternary carbon atoms (δc 113.7, 162.2, 114.5, 156.5, 113.4, 137.7, 136.5 and 135.0) and 1 carbonyl group (δc 193.3). Its 1H-NMR spectral data revealed the signal of one aldehyde proton (1-CHO, δH 10.13), one sp3 methyl attached to the aromatic ring (6-CH3, δH 2.59), four sp3 methyl groups (δH 1.77, 1.59, 1.15 and 1.19), one sp3 methine (δH 3.35), two sp2 methines (δH 5.19 and 5.15) and five sp3 methylenes (δH 3.39, 2.01, 2.09, 2.06 and 2.19).
Alignments of vicinal protons and carbons were determined by carrying out 1H–1H COSY and heteronuclear multiple bond correlation (HMBC) experiments. 1H–1H COSY experiments revealed correlations from 7-H to 9-CH3 through to 8-H, and from 10-H to 16-H through to 11-H, 12-H, 13-CH3, 14-H and 15-H, as indicated by the bold-faced lines in Figure 2. Assignments of the signals for nine quaternary carbons (C-1, C-2, C-3, C-4, C-5, C-6, C-9, C-13 and C-17) were revealed by HMBC experiments. The long-range couplings from the aldehyde proton 1-CHO to C-1, C-2 and C-3; from the methyl proton 6-CH3 to C-1, C-5 and C-6; from the methylene proton 7-H to C-2, C-3, C-4, C-8 and C-9; from the methine proton 8-H to C-7, 9-CH3 and C-10; from the methyl proton 9-CH3 to C-8, C-9 and C-10; from the methylene proton 10-H to C-9, C-11 and C-12; from the methylene proton 11-H to C-10, C-12 and C-13; from the methine proton 12-H to C-14; from the methyl proton 13-CH3 to C-12, C-13 and C-14; from the methyl proton 17-CH3 to C-16, C-17 and 17-CH3′; and from the methyl proton 17-CH3′ to C-16, C-17 and 17-CH3 were confirmed from HMBC spectra (Table 1).
In differential NOE experiments, the correlations among 8-H, 7-H and 10-H, and among 7-H, 9-CH3 and 13-CH3 were observed, and the stereochemistry of the double bond at C-8 was deduced to be E. Similarly, the correlations among 12-H, 11-H and 14-H, and among 9-CH3, 13-CH3 and 17-CH3′, established the stereochemistry of the double bond at C-12 to be E. The presence of a chlorine was shown using HRFAB-MS analysis, and the connection of its atom to C-5 was reasonable from its chemical shift (δc 113.4).
Biological activity
It is reported that ascochlorin inhibits the growth of yeast C. albicans, in addition to having antiviral activity.1 Ascochlorin at a concentration of 5 mg ml−1 (12.4 mM) caused a clear growth inhibitory zone (ϕ 14 mm) when tested against C. albicans NBRC 1386 in the paper-disc agar diffusion assay, but no such zone appeared with LL-Z1272α epoxide at a concentration of 12.3 mM. The diameter of the paper disc was 8 mm.
Discussion
The parent strain, A. viciae Libert, concurrently produces both ascochlorin and ascofuranone.2 There are many structurally related compounds with various biological activities (see Introduction), but studies on their biosyntheses have not been reported yet. We obtained mutants that produced LL-Z1272α and/or LL-Z1272α epoxide, which is likely to be a precursor of ascochlorin. Mutant J-15 produced LL-Z1272α only (unpublished data), whereas mutant J-29 produced LL-Z1272α epoxide, together with ascofuranone (in this report). Both ascochlorin and LL-Z1272α have been isolated together from Fusarium species,3 Nectria coccinea6 and Verticillium sp.10 Ellestad et al.3 proposed that the farnesyl chain of LL-Z1272α was epoxidized, cyclized to a cyclohexanone ring and converted to ascochlorin (Figure 3), just as in the case of the enzymatic conversion of squalene 2, 3-oxide to lanosterol and cholesterol.19 However, the epoxide, the expected intermediate to which the farnesyl chain with a terminal double bond was converted, had not been previously isolated. The isolation of LL-Z1272α epoxide supports the hypothesis of Ellestad et al., with the epoxide being converted into ascochlorin similarly as squalene oxide is converted into sterols. Thus, we are convinced that LL-Z1272α epoxide is a precursor of ascochlorin. Mutant J-29 produced the epoxide along with ascofuranone in an amount that was approximately one-half of that of the epoxide, suggesting that the activity of converting the epoxide to ascochlorin was severely blocked in the mutant and that the conversion into ascofuranone was less obstructed in it. If there were these branching pathways from the LL-Z1272α epoxide, the yield amounts of ascochlorin and ascofuranone might roughly correspond to the activity of each of the converting enzymes. In the future, it will be necessary to verify whether LL-Z1272α is converted into its epoxide by a specific enzyme and then to investigate whether the epoxide is converted into ascochlorin and ascofuranone separately by different branches of the biosynthetic pathway.
Methods
Mutation and isolation of mutants
A. viciae Libert isolated from soil1 was used as the parent strain and was maintained on a malt extract-yeast extract (MY) agar medium composed of glucose 1%, polypeptone 0.5%, yeast extract 0.3% and malt extract 0.3% at pH 6 in agar 1.8%. The strain grown on the MY agar medium at 28 °C for 7 days was harvested by centrifugation at 7300 g for 10 min and was washed once with 0.9% (w/v) NaCl solution. The short mycelial cells were suspended in the saline containing N-methyl-N′-nitro-N-nitrosoguanidine (NTG) at a final concentration of 10, 30 or 100 μg l–1. After each cell suspension had been incubated with one of the above concentrations of NTG for 60 min at 28 °C, the cell was harvested by centrifugation, washed twice with saline, suspended in MY medium and incubated at 28 °C with gentle shaking for 2 h. Each cell suspension was appropriately diluted with saline and spread on the MY agar medium. After incubation at 28 °C for 7 days, a single colony from the colonies that appeared on the medium was transferred to the MY agar medium and maintained. About 400 mutant colonies were isolated with a certain concentration of NTG. For screening, the isolated fungal mutant was cultured in a thick test tube (ϕ 25 mm × 20 cm) containing 20 ml of production medium (see following part), and the mycelium of each mutant strain was extracted with four volumes of MeOH to analyze for the presence of new metabolites. The production of new metabolites was monitored by HPLC. Each mutant that produced new ascochlorin-related compounds was repeatedly treated with different concentrations of NTG to generate further mutants that produced larger quantities of the new metabolites. The mutant J-29 was finally obtained after the parent strain had been mutated with successive concentrations of 10, 100, 100, 30 and 100 μg l−1 NTG. Therefore, the mutant J-29 was selected from more than 2000 colonies.
Fermentation of A. viciae mutant J-29
For production of LL-Z1272 α epoxide, the mutant J-29 was cultured in production medium composed of glucose 7.0%, polypeptone 0.3%, yeast extract 0.2%, KH2PO4 0.05%, MgSO4·7H2O 0.05%, corn steep liquor 0.1%, (NH4)2SO4 0.1%, CaCl2·2H2O 0.2%, CH3COONa 0.5%, CaCO3 0.1% and one drop of Adekanol LG-109 (anti-foaming agent; Adeka, Tokyo, Japan). The fungus was aerobically cultured at 28 °C for 7 days in a 500-ml Erlenmeyer flask containing 50 ml of the production medium.
HPLC analysis
To analyze the fungal products of isolated mutant strains, we cultured each mutant at 28 °C for 7 days in a 500-ml Erlenmeyer flask containing 50 ml of production medium. The mycelium of each mutant was harvested by centrifugation at 7300 g for 10 min and extracted with four volumes of MeOH. The extract was concentrated under reduced pressure and dissolved in a small amount of MeOH for analysis of products using a Shimadzu HPLC system, Model LC-VP (Shimadzu, Kyoto, Japan), equipped with an ODS column (YMC-Pack ODS-A302; 150 × 4.6 mm i.d.; YMC, Kyoto, Japan). The column was maintained at 40 °C and eluted isocratically with a mixture of acetonitrile–isopropyl alcohol–H2O–acetic acid (350:200:450:2, v/v) at a flow rate of 2.0 ml min−1. A small portion of the MeOH extract was subjected to HPLC using an automatic injection system, and separation was monitored at 295 nm. Representative HPLC chromatograms are shown in Figure 4. Ascochlorin and ascofuranone were determined by comparison with authentic compounds.
Isolation and purification of LL-Z1272α epoxide
The mutant J-29 was cultured in production medium (4500 ml) and its mycelium was extracted with four volumes of MeOH. The extract was concentrated in vacuo and dissolved in brine. Thereafter, ascochlorin and its structurally related compounds were extracted with EtOAc and concentrated in vacuo. The extract (3.52 g) was passed through a silica gel 60 column (ϕ 35 mm × 13 cm) with a mixture of EtOAc and n-hexane (1:5, v/v). The fraction eluted with the mixture of solvents (2.56 g) was passed again through another silica gel 60 column (ϕ 50 mm × 26 cm), and four fractions, 1.35 g, 0.11 g, 0.74 g and 0.23 g, in the order eluted, were separated by the same mixture of EtOAc and n-hexane (1:5, v/v). The first fraction (1.35 g) was purified repeatedly by silica gel 60 column chromatography and was crystallized with difficulty. Finally, crystals with a pale yellow color (1.1 g) were obtained. From 1H-NMR and 13C-NMR analyses, the substance was elucidated to be LL-Z1272α epoxide. The second and fourth fractions were not analyzed further. The third fraction (0.74 g) was identical to ascofuranone on the basis of comparison of 1H-NMR spectra and the retention time of authentic ascofuranone on the HPLC chromatogram. After the fractions had been separated by silica gel 60 thin-layer chromatography with a mixture of EtOAc and n-hexane (1:5, v/v), the Rf values for ascochlorin, ascofuranone and LL-Z1272α epoxide were calculated to be 0.19, 0.25 and 0.31, respectively.
Spectroscopic measurements
NMR spectra were recorded on a Jeol JNM-ECA 500 FT NMR system (Jeol, Tokyo, Japan), with 1HNMR at 500 MHz and 13C–NMR at 125 MHz, using deuterated solvent (CDCl3). The melting point value was uncorrected. UV spectra were recorded on a Shimadzu UV-160A spectrophotometer, and IR spectra were measured with a Jasco FT/IR-7300 spectrometer linked to a WS/ IR-7300 workstation (Jasco, Tokyo, Japan). Mass spectra were recorded with a Jeol SX-102A spectrometer in the FAB mode by using glycerol as matrix and polyethylene glycol as the internal standard. Optical rotation values were recorded with a Jasco DIP-1000 polarimeter.
Inhibitory activity against Candida albicans
For determination of the inhibitory activity against C. albicans NBRC 1386, each solution of ascochlorin and LL-Z1272α epoxide, with the same concentration of 5 mg ml−1, was applied to a paper disc (ϕ 8 mm), and each paper disc was then placed onto YPD agar medium onto which C. albicans had been spread. The YPD medium was composed of yeast extract 1%, peptone 2% and glucose 2% at pH 7.2 in agar 1.8%.
References
Tamura, G., Suzuki, S., Takatsuki, A., Ando, K. & Arima, K. Ascochlorin, a new antibiotic, found by paper-disc agar-diffusion method. I. Isolation, biological and chemical properties of ascochlorin (Studies on antiviral and antitumor antibiotics. I). J. Antibiot. 21, 539–544 (1968).
Sasaki, H., Okutomi, T., Hosokawa, T., Nawata, Y. & Ando, K. Ascofuranone, a new antibiotic from Ascochyta viciae. Tetrahedron Lett. 25, 2541–2544 (1972).
Ellestad, G. A., Evans, R. H. Jr & Kunstmann, M. P. Some new terpenoid metabolites from an unidentified Fusarium species. Tetrahedron 25, 1323–1334 (1969).
Kato, A., Ando, K., Tamura, G. & Arima, K. Cylindrochlorin, a new antibiotic produced by Cylindrocladium. J. Antibiot. 23, 168–169 (1970).
Hayakawa, S., Minato, H. & Katagiri, K. The ilicicolins, antibiotics from Cylindrocladium ilicicola. J. Antibiot. 24, 653–654 (1971).
Aldridge, D. C. et al. Metabolites of Nectria coccinea. J. Chem. Soc. [Perkin I] 17, 2136–2141 (1972).
Kosuge, Y., Suzuki, A., Hirata, S. & Tamura, S. Structure of colletochlorin from Colletotrichum nicotianae. Agric. Biol. Chem. 37, 455–456 (1973).
Cagnoli-Bellavita, N., Ceccherelli, P., Fringuelli, R. & Ribaldi, M. Ascochlorin: a terpenoid metabolite from Acremonium luzulae. Phytochemistry 14, 807 (1975).
Kawagishi, H., Sato, H., Sakamura, S., Kobayashi, K. & Ui, T. Isolation and structure of a new diprenyl phenol, colletorin B produced by Cephalosporium diospyri. Agric. Biol. Chem. 48, 1903–1904 (1984).
Takamatsu, S. et al. A novel testosterone 5α-reductase inhibitor, 8′,9′-dehydroascochlorin produced by Verticillium sp. FO-2787. Chem. Pharm. Bull 42, 953–956 (1994).
Singh, S. B. et al. Chemistry and biology of cylindrols: novel inhibitors of Ras farnesyl-protein transferase from Cylindrocarpon lucidum. J. Org. Chem. 61, 7727–7737 (1996).
Che, Y., Swenson, D. C., Gloer, J. B., Koster, B. & Malloch, D. Pseudodestruxins A and B: new cyclic depsipeptides from the coprophilous fungus Nigrosabulum globosum. J. Nat. Prod. 64, 555–558 (2001).
Seephonkai, P., Isaka, M., Kittakoop, P., Boonudomlap, U. & Thebtaranonth, Y. A novel ascochlorin glycoside from the insect pathogenic fungus Verticillium hemipterigenum BCC 2370. J. Antibiot. 57, 10–16 (2004).
Hosokawa, T., Sawada, M., Ando, K. & Tamura, G. Enhanced excretion of fecal neutral sterols and the hypocholesterolemic property of 4-O-methylascochlorin in mice. Agric. Biol. Chem. 44, 2461–2468 (1980).
Hosokawa, T., Okutomi, T., Sawada, M., Ando, K. & Tamura, G. Unusual concentration of urine and prevention of polydipsia by fungal prenylphenols in DOCA hypertensive rats. Eur. J. Pharmacol. 69, 429–438 (1981).
Hosokawa, T., Ando, K. & Tamura, G. An ascochlorin derivative, AS-6, potentiates insulin action in streptozotocin diabetic mice and rats. Agric. Biol. Chem. 46, 2865–2869 (1982).
Magae, J., Hosokawa, T., Ando, K., Nagai, K. & Tamura, G. Antitumor protective property of an isoprenoid antibiotic, ascofuranone. J. Antibiot. 35, 1547–1552 (1982).
Magae, J. et al. Antitumor and antimetastatic activity of an antibiotic, ascofuranone, and activation of phagocytes. J. Antibiot. 41, 959–965 (1988).
Willett, J. D., Sharpless, K. B., Lord, K. E., van Tamelen, E. E. & Clayton, R. B. Squalene-2,3-oxide, an intermediate in the enzymatic conversion of squalene to lanosterol and cholesterol. J. Biol. Chem. 242, 4182–4191 (1967).
Acknowledgements
We are very grateful to Dr K Ando for providing A. viciae Libert and authentic compounds, ascochlorin and ascofuranone. We also thank Dr H Kondo (NRL Pharma) for discussion on the isolation of the epoxide. This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosono, K., Ogihara, J., Ohdake, T. et al. LL-Z1272α epoxide, a precursor of ascochlorin produced by a mutant of Ascochyta viciae. J Antibiot 62, 571–574 (2009). https://doi.org/10.1038/ja.2009.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2009.80
Keywords
This article is cited by
-
Re-identification of the ascofuranone-producing fungus Ascochyta viciae as Acremonium sclerotigenum
The Journal of Antibiotics (2017)
-
Ascochlorin derivatives from the leafhopper pathogenic fungus Microcera sp. BCC 17074
The Journal of Antibiotics (2015)