Abstract
The macrolide antibiotic azithromycin has an antiproliferative and autophagic effect on rabbit tracheal smooth muscle cells (SMCs). The purpose of this study is to investigate the effect of azithromycin on human bronchial SMCs. Human bronchial SMCs were treated with azithromycin (10−5 M) in the presence or absence of 10% fetal bovine serum (FBS). Cell number was estimated using the Cell Titer 96 AQueous One Solution Assay. Induction of autophagy was studied by observation of cell morphology in cells treated or not with the autophagy inhibitor, 3-methyladenine (3-MA), as well as by Lysotracker Red staining of lysosomes. Activation of apoptosis was assessed with flow cytometry after annexin staining. Incubation with azithromycin for 24, 48 or 72 h reduced viability in FBS-deprived cells, as well as cells cultured in FBS-containing medium. Azithromycin treatment resulted in the formation of cytoplasmic vacuoles that could not be prevented by 3-MA. Furthermore, 3-MA did not reverse the effect of azithromycin on the viability of SMCs. There was an increase in the number of lysosomes in cells treated with azithromycin. Finally, azithromycin increased the percentage of early apoptotic cells. In conclusion, azithromycin reduces the viability of human bronchial SMCs possibly by leading to apoptotic cell death.
Similar content being viewed by others
Introduction
Azithromycin is a widely used and effective antibiotic because of its antibacterial and anti-inflammatory actions. In airway diseases, macrolide therapy seems to be very effective. Specifically, azithromycin is used in low dosages, for a long time, in chronic airway diseases such as chronic bronchitis, diffuse panbronchiolitis and bronchial asthma.1, 2, 3, 4, 5 Airway wall remodeling, which appears in some of these conditions, includes among others, thickening of the reticular membrane, proliferation of the smooth muscle cells (SMCs) and increase in both the number and size of vessels.6, 7
The improvement in the health status of patients treated with macrolides was attributed to the bacteriostatic and anti-inflammatory effects of such antibiotics. However, data available from previous studies in our laboratory show that azithromycin relaxes precontracted rabbit airway smooth muscle.8 Furthermore, we have shown that azithromycin causes a dose-dependent reversible reduction of SMCs proliferation accompanied by autophagy in cells isolated from the trachea of adult rabbits.9
The purpose of this study is to examine whether azithromycin has the same effect on human bronchial SMCs.
Materials and Methods
Culture of airway SMCs
Human bronchial SMCs (cc-2576, Lonza Group, Basel, Switzerland) were used. Cells were grown in Dulbecco's modified Eagle's medium/Ham/F12 (DMEM/F12) containing 10% fetal bovine serum (FBS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Gibco BRL, Gaithersburg, MD, USA; Invitrogen Corporation, Carlsbad, CA, USA) at 37 °C in a humidified incubator under 5% CO2. All experiments were carried out with cells on passages 3–6.
Treatment of cells
Cells were cultured in serum-free medium for 72 h before further treatment to achieve quiescence. Azithromycin (10−5 M) (Sigma-Aldrich Chemie, Steinheim, Germany) and 10% FBS were added to cells for 24–72 h as indicated. Control cells remained in serum-free medium. Azithromycin was dissolved in DMEM/F12 medium.
Induction of autophagy
The autophagy inhibitor 3-methyladenine (10 mM) (3-MA, Sigma-Aldrich Chemie) was added to the medium 3 h before the addition of azithromycin. 3-MA was dissolved in water. The cells were observed under a Nikon eclipse TS100 (Nikon, Melville, NY, USA) inverted microscope using a Leica DFC 480 camera (Leica Cameras, Solms, Germany).
Cell number estimation
Cells were seeded into 96-well plates at a density of 2000 cells per well, allowed to adhere overnight, washed twice with phosphate-buffered saline and incubated in serum-free medium for 72 h before further treatment. Replicates of three were used for each point. Cell number was assessed using the Cell Titer 96 AQueous One Solution Assay (Promega, Madison, WI, USA). Briefly, MTS tetrazolium reagent was added directly to each well and incubated for 2 h at 37 °C. The MTS tetrazolium compound is converted to formazan by living cells.10, 11 Absorbance was measured at 490 nm with a reference at 630 nm in an ELISA plate reader. There was a linear response between cell number and absorbance at 490 nm (data not shown).
Lysotracker Red staining
Cells were stained with Lysotracker Red (Molecular Probes, Eugene, OR, USA; Invitrogen Corporation).12 Briefly, cells were incubated in 50 nM Lysotracker Red for 2 h at 37 °C, 5% CO2, mounted in a drop of phosphate-buffered saline and observed under a Zeiss Axioskop 40 microscope (Carl Zeiss, New York, NY, USA), using a Leica DFC 480 camera (Leica Cameras).
Assessment of apoptosis
To obtain the proportion of early apoptotic, alive and dead cells, SMCs were stained with Annexin V and 7-AAD using the Beckman Coulter Annexin V-FITC/7-AAD Kit (Beckman Coulter, Fullerton, CA USA). Accordingly, cells were harvested, washed twice with ice-cold 1 × phosphate-buffered saline, cell pellets resuspended in 100 μl ice-cold 1 × binding buffer, and 10 μl of Annexin V-FITC solution and 20 μl of 7-AAD Viability Dye were added. Cells were mixed gently, incubated for 15 min in the dark on ice and events were acquired using an FC500 flow cytometer system (Beckman Coulter) equipped with an argon ion laser and analyzed on the FL1 and FL3 channels. Fluorescence thresholds were determined using unstained and single stained cell populations.
Analysis
In cell viability experiments, each point was carried out in triplicate and the values presented are the mean of independent experiments. All data are expressed as means±s.e. and N refers to the number of experiments. Differences between means were analyzed by one-way analysis of variance with statistically significant differences between groups being determined by Bonferroni post hoc test. A comparison is considered significant when the P-value is <0.05. The statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). Image analysis of the images obtained from the Lysotracker Red staining was conducted using Adobe Photoshop 7.0 (www.adobe.com/products/photoshop/family), and results were expressed as intensity values.
Results
Incubation with 10−5 M azithromycin for 24–72 h of quiescent SMC in serum-free medium significantly reduced the human bronchial SMC number. For example, the number of SMCs in the presence of azithromycin was 59.3±12.5% at 24 h, 51.7±6.5% at 48 h and 56.2±6.2% of control at 72 h (P<0.01 compared with control, Figure 1).
The number of human bronchial SMCs treated with 10−5 M azithromycin in the presence of 10% FBS for 24, 48 and 72 h was also suppressed to 54.2±6.31, 46.4±1.9 and 49.5±4.0% of control, respectively (Figure 1). On the other hand, the estimated cell number in 10% FBS was increased to 118.7±5.2% at 24 h, 141.2±9.1% at 48 h and 157.4±9.4 at 72 h compared with control (Figure 1).
Treatment of human bronchial SMCs, with 10−6–6 × 10−6 M azithromycin in 10% FBS-containing or in serum-free medium, did not significantly affect their number (data not shown).
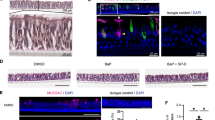
Moreover, treatment of human bronchial SMCs with 10−5 M azithromycin for 24 h both in the presence or absence of FBS resulted to the formation of cytoplasmic vacuoles, reminiscent of autophagy (Figure 2).
We assessed whether 3-MA (10 mM), an inhibitor of class III phosphotidylinositol-3-kinases that blocks autophagy,13, 14 inhibits the effect of azithromycin in these cells. Pretreatment of human bronchial SMCs with 3-MA had no significant effect on the formation of vacuoles (Figure 2). In addition, 3-MA pretreatment did not affect the reduction of SMC numbers induced by azithromycin (Figure 3).
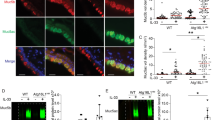
To study the nature of the vacuoles formed in azithromycin-treated cells, we used Lysotracker Red, a fluorescent acidotropic probe for labeling and tracking of lysosomes in live cells. The treatment of SMCs with 10−5 M azithromycin for 24 h increased the luminosity intensity level (Figure 4), indicating an increase in the number of lysosomes.
Finally, the proportion of early apoptotic, alive and dead cells was studied using Annexin V and 7-AAD staining. Azithromycin increased the percentage of the early apoptotic population from 14.5 to 27.5% when added to cells in the FBS-free medium and from 6.8 to 20.1% in cells cultured in FBS-containing medium (Figure 5).
Discussion
The results from this study indicate that azithromycin reduces the viability of human bronchial airway SMCs. This effect is probably mediated by the induction of autophagy that drives the cells to apoptosis. Azithromycin decreases the viability of human bronchial SMCs cultured both in medium containing 10% FBS, as well as in serum-free medium. These results suggest that the effect of azithromycin on these cells is independent of the proliferative status of the cells. Moreover, it does not depend on the duration of treatment with azithromycin, as the effect reaches its maximum at the first 24 h of incubation in both FBS-fed and serum-starved cells.
There are studies showing that in chronic airway diseases SMC represent a mixed population of two different phenotypes, a contractile (elongated cells) and a synthetic one. These two phenotypes seem to have different responses to stimuli that affect cell proliferation.15, 16, 17 The percentage of the contractile phenotype in canine tracheal SMCs is about 8.9%, and after 7 days of serum starvation it appears elevated to the point of 18% of total population.17 Therefore, only a minor percentage of the culture population seems to be different. In our experiments, we used cells that were incubated in serum-free medium for 72 h to synchronize the cells in a nonproliferative status. The population we used seems to be homogeneous. The lack of time-dependent effect could be attributed to the fact that azithromycin is a stimulus too intense for these cells, which leads them rapidly to apoptosis after 24 h of treatment.
There are a few studies on epithelial or SMCs that suggest that macrolide antibiotics may affect cell proliferation. Specifically, roxithromycin, SAR943, a rapamycin analog, as well as rapamycin itself inhibit the proliferation of human coronary artery SMCs,18 epithelial and airway SMCs,19 and rapamycin also inhibits the proliferation of hepatocytes.20 Other macrolides such as erythromycin have been shown to inhibit hypertrophic and metaplastic changes of goblet cells in rat nasal epithelium, as well as inhibit the proliferation of human mononuclear cells.21, 22 Azithromycin along with clarithromycin are reported to induce apoptosis of activated lymphocytes.23 In previous studies in our laboratory, azithromycin was also observed to cause reversible autophagy in rabbit tracheal SMCs, but not apoptosis, as well as reversible inhibition of the proliferation.9 Interestingly, azithromycin seems to affect cell proliferation by inducing both autophagy and apoptosis, with the threshold between the two procedures being affected by cell type.
Azithromycin treatment caused cytoplasmic vacuoles formation. These vacuoles are stained with Lysotracker Red, pointing out the presence of lysosomes and seem to be autophagic. However, the inhibitor of autophagy, 3-MA, did not prevent neither vacuole formation nor the reduction of cell number, caused by azithromycin. Moreover, the percentage of early apoptotic cells increased after azithromycin treatment. These results suggest that azithromycin may induce an intense or fast effect on human bronchial SMCs that leads the cells to extended autophagy and induces apoptosis within the first 24 h of treatment. Previous studies in our laboratory showed that azithromycin caused a reversible inhibition of rabbit tracheal SMCs proliferation, accompanied by autophagy, but did not induce apoptosis.9 However, there is evidence that autophagy and apoptosis are not mutually exclusive.24, 25, 26 Whether stress conditions will lead the cell to autophagy or apoptosis depends on the nature, intensity and duration of the effect, as well as the cell type and energy condition of the cell at the time that it received the effect.27
Furthermore, although azithromycin displayed a dose-response effect on rabbit tracheal SMC proliferation, in concentrations 10−6–10−5 M,9 such a dose-dependent effect could not be observed on human bronchial SMCs. In contrast, azithromycin seems to have a none or all effect in human bronchial SMCs, with the concentration 10−5 M being the threshold. Interestingly, when azithromycin has been used in clinical studies, the administrated concentration resulted in a lung concentration of 8.93 mg l−1, corresponding to ∼10−5 M of azithromycin.28, 29 The same concentrations were used when the anti-inflammatory and antibacterial effect of azithromycin was studied.30, 31
In summary, our results show that treatment of human SMCs with azithromycin reduces cell viability possibly through apoptosis. In addition to the known antibacterial and anti-inflammatory role of azithromycin, this pro-apoptotic effect on human bronchial SMCs might be of clinical significance.
References
Kudoh, S., Azuma, A., Yamamoto, M., Izumi, T. & Ando, M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157, 1829–1832 (1998).
Oishi, K. et al. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airway of patients with chronic airway diseases. Infect. Immun. 62, 4145–4152 (1994).
Shirai, T., Sato, A. & Chida, K. Effect of 14-membered ring macrolide therapy on chronic respiratory tract infections and polymorphonuclear leukocyte activity. Intern. Med. 34, 469–474 (1995).
Miyatake, H. et al. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest 99, 670–673 (1991).
Cazzola, M., Salzillo, A. & Diamare, F. Potential role of macrolides in the treatment of asthma. Monaldi. Arch. Chest Dis. 55, 231–236 (2000).
Vignola, A. et al. Airway remodelling in asthma. Chest 123, 417S–422S (2003).
Jeffery, P. K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 164, S28–S38 (2001).
Daenas, C., Hatziefthimiou, A. A., Gourgoulianis, K. I. & Molyvdas, P. A. Azithromycin has a direct relaxant effect on precontracted airway smooth muscle. Eur. J. Pharmacol. 553, 280–287 (2006).
Stamatiou, R. et al. Azithromycin has an antiproliferative and autophagic effect on airway smooth muscle cells. Eur. Respir. J. 34, 1–11 (2009).
Cory, A. H., Owen, T. C., Barltrop, J. A. & Cory, J. G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3, 207–212 (1991).
Berridge, M. V. & Tan, A. S. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482 (1993).
Shen, Z. Y. et al. Autophagy and endocytosis in the amnion. J. Struct. Biol. 162, 197–204 (2008).
Seglen, P. O. & Gordon, P. B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl Acad. Sci. USA 79, 1889–1892 (1982).
Petiot, A., Ogier-Denis, E., Blommaart, E. F., Meijer, A. J. & Codogno, P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signalling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 (2000).
Hirst, S. J., Walker, T. R. & Chilvers, E. R. Phenotypic diversity and molecular mechanisms of airway smooth muscle proliferation in asthma. Eur. Respir. J. 16, 159–177 (2000).
Bentley, J. K. & Hershenson, M. B. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc. Am. Thorac. Soc. 5, 89–96 (2008).
Halayko, A. J. & Amrani, Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Physiol. Neurobiol. 137, 209–222 (2003).
Tomita, H. et al. Roxithromycin is an inhibitor of human coronary artery smooth muscle cells proliferation: a potential ability to prevent coronary heart disease. Atherosclerosis 182, 87–95 (2005).
Fujitani, Y. & Trifilieff, A. In vivo and in vitro effects of SAR 943, a rapamycin analogue, on airway inflammation and remodeling. Am. J. Respir. Crit. Care Med. 167, 193–198 (2003).
Buitrago-Molina, L. E. et al. Rapamycin delays tumor development in murine livers by inhibiting proliferation of hepatocytes with dna damage. Hepatology 50, 2 (2009).
Takahashi, Y., Shimizu, T. & Sakakura, Y. Effects of indomethacin, dexamethasone, and erythromycin on endotoxin-induced intraepithelial mucus production of rat nasal epithelium. Ann. Otol. Rhinol. Laryngol. 106, 683–687 (1997).
Roche, Y., Gougerot-Pocidalo, M. A., Fay, M., Forest, N. & Pocidalo, J. J. Macrolides and immunity: effects of erythromycin and spiramycin on human mononuclear cell proliferation. J. Antimicrob. Chemother. 17, 195–203 (1986).
Mizunoe, S. et al. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. Int. Immunopharmacol. 4, 1201–1207 (2004).
Cui, Q., Tashiro, S., Onodera, S., Minami, M. & Ikejima, T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol. Pharm. Bull. 30, 859–864 (2007).
Yan, C. H. et al. Autophagy is involved in cytotoxic effects of crotoxin in human breast cancer cell line MCF-7 cells. Acta. Pharmacol. Sin. 28, 540–548 (2007).
Shacka, J. et al. Bafilomycin A1 inhibits cloroquine-induced death of celebellar granule neurons. Mol. Pharmacol. 69, 1125–1136 (2006).
Canu, N. et al. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J. Neurochem. 92, 1228–1242 (2005).
Shimizu, T., Shimizu, S., Hattori, R., Gabazza, E. C. & Majima, Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am. J. Respir. Crit. Care Med. 168, 581–587 (2003).
Danesi, R. et al. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 51, 939–945 (2003).
Patel, K. B. et al. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40, 2375–2379 (1996).
Phaff, S. J., Tiddens, H. A., Verbrugh, H. A. & Ott, A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J. Antimicrob. Chemother. 57, 741–746 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stamatiou, R., Boukas, K., Paraskeva, E. et al. Azithromycin reduces the viability of human bronchial smooth muscle cells. J Antibiot 63, 71–75 (2010). https://doi.org/10.1038/ja.2009.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2009.125
Keywords
This article is cited by
-
Effects of azithromycin on bronchial remodeling in the natural model of severe neutrophilic asthma in horses
Scientific Reports (2022)
-
Azithromycin suppresses CD4+ T-cell activation by direct modulation of mTOR activity
Scientific Reports (2014)