Abstract
Light underpins the health and function of coral reef ecosystems, where symbiotic partnerships with photosynthetic algae constitute the life support system of the reef. Decades of research have given us detailed knowledge of the photoprotective capacity of phototrophic organisms, yet little is known about the role of the host in providing photoprotection in symbiotic systems. Here we show that the intracellular symbionts within the large photosymbiotic foraminifera Marginopora vertebralis exhibit phototactic behaviour, and that the phototactic movement of the symbionts is accomplished by the host, through rapid actin-mediated relocation of the symbionts deeper into the cavities within the calcium carbonate test. Using a photosynthetic inhibitor, we identified that the infochemical signalling for host regulation is photosynthetically derived, highlighting the presence of an intimate communication between the symbiont and the host. Our results emphasise the central importance of the host in photosymbiotic photoprotection via a new mechanism in foraminifera that can serve as a platform for exploring host–symbiont communication in other photosymbiotic organisms.
Similar content being viewed by others
Introduction
The benthic foraminifera Marginopora vertebralis Quoy and Gaimard, 1830, is a large, single-celled, calcifying microorganism belonging to the infrakingdom Rhizaria. It is typically found in coral reef ecosystems, where it is a major contributor to calcite export from the surface waters to the reef structure, with calcium carbonate tests (calcite skeletons) often dominating the sediment (Langer et al., 1997; Doo et al., 2012). M. vertebralis forms a symbiotic partnership with one of the most important symbiotic algal species in tropical reef systems, the dinoflagellate Symbiodinium (Pawlowski et al., 2001), renowned for living in endosymbioses with reef-building corals across the globe (Baker, 2003). This partnership has evolved to make use of the abundance of light in the clear, nutrient-poor waters of the reef; whereby the host receives energy from the photosynthetic symbiont in the form of fixed carbon (Lee, 2006) in exchange for providing the symbiont with access to a rich supply of inorganic nutrients. Although light underpins the health and function of coral reef ecosystems, in excess, light can result in reduced photosynthetic efficiency, and if not protected against, damage to the photosynthetic machinery of the symbiont can ensue (Brown et al., 1999; Jones and Hoegh-Guldberg, 2001). Therefore, the success of photosymbiotic partnerships relies on the ability for the symbiont and host to regulate incoming irradiance with nutrient acquisition, serving two purposes: (1) to optimise carbon productivity by the symbionts, ultimately benefitting the host, and (2) to minimise the production of reactive oxygen species, which may damage both symbiont and host.
The ubiquity of Symbiodinium in reef symbioses has resulted in extensive research into understanding light regulation and stress responses in these microalgae (Iglesias-Prieto and Trench, 1994; Iglesias-Prieto and Trench, 1997; Jones and Hoegh-Guldberg, 2001). In corals—the most extensively studied photosymbiotic system in tropical reefs—photoprotection is primarily regulated by the Symbiodinium, which have evolved mechanisms to dissipate excess energy as heat and thus protect their photosystems from damage (Brown et al., 1999). It has also been shown that the coral host can contribute to light protection via accumulation of fluorescent proteins that absorb light in the harmful wavelengths (Salih et al., 2000; Dove et al., 2008), or more directly via contraction or expansion of tissue, which modulates the light field around the symbionts within specific tissue layers (Brown et al., 2002; Dimond et al., 2012; Wangpraseurt et al., 2014). Similar to corals, M. vertebralis is often found in shallow, well-lit waters of the sandy reef sediment (Sinutok et al., 2011) and, therefore, must balance incoming energy with photoprotection. Unlike corals, however, M. vertebralis are motile and as such can achieve photoprotection through relocation to more shaded habitats. Indeed, M. vertebralis has been shown to exhibit negative phototaxis; moving into a shaded environment when exposed to high light, a response proposed to be driven by the light-sensitive symbionts (Sinutok et al., 2013). Their movement is, however, relatively slow (up to 8 mm h−1) (Khare and Nigam, 2000) and thus ineffective in providing immediate protection from damaging irradiances once exposed. One study has reported a different sort of phototaxis in stationary M. vertebralis, where the symbionts were observed to move vertically within the calcified test from the darker underside to the illuminated top-side (Ross, 1972). Although not examined in any detail, this movement was assumed to be the result of flagellated Symbiodinium swimming towards the light inside the host test. Here we investigate whether intracellular phototaxis could be used as a means of photoprotection. We show that vertical migration of symbionts within M. vertebralis serves to rapidly and effectively protect the intracellular symbionts under high-light stress. This mode of symbiont migration represents a novel mechanism in which the phototaxis of the symbionts is host-mediated; the relocation of the symbionts being accomplished through host-derived actin filament contraction as opposed to driven by flagellated movement of the symbionts, as was previously assumed. Our study reveals a novel regulatory mechanism for host-mediated photoprotection that may serve as a platform for studying host–symbiont communication in other photosymbiotic organisms such as corals, providing a new means for investigating signalling between the ubiquitous Symbiodinium algae and its host.
Materials and methods
Sample collection and experimental design
Individual specimens of M. vertebralis were collected from the inner reef flat of Heron Island, Great Barrier Reef, Australia (July 2014) and maintained at 22 °C in aquaria with flow-through artificial sea water on a 12:12 h (light:dark) cycle for several weeks prior to the experiment. Light was supplied from a programmable blue/white LED panel (2-channel Phantom, CIDLY Ltd, Shenzhen, China) providing a coarse sinusoidal light cycle (16-step light levels) with a midday maximum of 130 μmol photons m−2 s−1. To investigate intracellular phototaxis in M. vertebralis, foraminifera were transferred into small beakers with 100 ml of artificial sea water and placed into two temperature-controlled water baths (maintained at 22 °C) and left for 1 h prior to initial measurements (T0). The light treatment consisted of incremental increases every hour (from 130 to 200, 400 and 800 μmol photons m−2 s−1), followed by a recovery period at 130 μmol photons m−2 s−1. Control incubations were kept at 130 μmol photons m−2 s−1 throughout the experiment. Light levels were selected based on the mean minimum saturating irradiance (108±4 μmol photons m−2 s−1) and photoinhibiting irradiance (301±12 μmol photons m−2 s−1) determined from steady-state light curves (rETR vs PAR) performed on individuals of M. vertebralis (n=6) prior to the experiment (Supplementary Figure S1). The experiment was repeated using 5 μg ml−1 of the actin filament inhibitor cytochalasin B (n=5–8) and to investigate the effect of 10 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (n=6–8). In both cases DMSO was added to the controls at the same concentration (0.1% v/v). At each time point (T0–T4), chlorophyll a fluorescence, colour change and reflectance were measured (see below) and individuals were sampled for pigment analyses and histological sectioning.
Symbiont photosystem activity and photoprotective pigments
Photosynthetic efficiency of the algal symbionts was measured on the surface and underside of the foraminifera (n=8) via chlorophyll a fluorescence using a pulse amplitude modulated (PAM) fluorometer (Imaging-PAM, MAXI version, Walz GmbH, Effeltrich, Germany). At each time point, the beaker containing the foraminifera was transferred to the PAM and a saturating pulse of light (saturating pulse width=0.8 s; saturating pulse intensity >3000 μmol photons m−2 s−1) applied to determine minimum (FO') and maximum fluorescence (FM'). Individuals were then carefully flipped using forceps and the underside measured before being returned to their original orientation and placed back into the incubation bath. From these two parameters the effective quantum yield of PSII was calculated as ΔF/FM'=(FM'−FO')/FM' (Schreiber, 2004). In addition, prior to the experiment, foraminifera were dark-adapted for 30 min and FO and FM recorded to calculate FV/FM as (FM−FO)/FM (Schreiber, 2004). This was repeated at the end of the experiment in both control- and light-treated foraminifera, to measure recovered FV/FM. As a measure of photosynthetic performance at each specific irradiance, excitation pressure over PSII (QM) was calculated as 1- (ΔF/FM'/FV/FM) (Iglesias-Prieto et al., 2004). As there was minimal spatial variability in fluorescence signal, all fluorescence values were therefore averaged across the organism. At each time point, three individuals from both light treatments were snap frozen in liquid nitrogen and stored at −80 °C for pigment processing. Individual foraminifera were extracted in chilled 100% acetone containing vitamin E and sonicated for 30 min in iced-water in the dark, then stored in the dark at 4 °C. After 24 h, 333 μl of polished water was added to reduce the acetone concentration to 90% v/v and sonicated for 15 min in iced-water. The foraminifer test was then removed and dried for area determination (see below). The acetone extracts were filtered directly into amber glass vials (Waters Australia Pty Ltd, Rydalmere, NSW, Australia) through a 0.2 μm PTFE 13 mm syringe filter (MicroAnalytix Pty Ltd, Taren Point, NSW, Australia) pre-wetted with acetone, and stored at −80 °C until analysis via high-performance liquid chromatography following the methods of van Heukelem and Thomas (2001). Pigments were identified by comparison of their retention times and spectra using calibration standards (DHI, Hørsholm, Denmark) and integrated using graphical software (Empower Pro, Waters Australia Pty Ltd). For area determination, each test was imaged and measured in ImageJ (Schneider et al., 2012), using the area integration function calibrated to a known standard.
Foraminifer colour change
Individuals were imaged with a digital microscope colour camera (MU500, Amscope, Irvine, CA, USA) attached to a dissection microscope (SM-6TY, Amscope). The colour intensity of each foraminifer was measured with ImageJ software (Schneider et al., 2012) by integrating the pixel intensity (whiteness) of the whole foraminifer. Pixel intensity was processed relative to the initial pixel intensity of each individual and only foraminifera with a uniform distribution of symbionts over their entire surface were included in the final data (minimum n=5).
Surface reflectance
Surface reflectance was measured using a polished glass fibre (Ocean Optics Inc., Dunedin, FL, USA) connected to a spectrophotometer (USB2000, Ocean Optics Inc.) using dedicated software (SpectraSuite, Ocean Optics Inc.). The glass fibre was positioned at a fixed distance and angle (45°) from the foraminifera surface using a manual micromanipulator (Unisense, Aarhus, Denmark). Reflectance was recorded for each individual (n=8) and the resultant spectra were standardised against absolute reflectance (white diffuse reflectance standard, Spectralon SRS-99, LabSphere Inc., North Sutton, UK).
Histology
Foraminifera were fixed in 1 ml 2.5% glutaraldehyde in phosphate buffered saline (1 × PBS; NaCl: 8.0, KCl: 0.2, Na2HPO4: 1.44, KH2PO4: 0.25 g l−1) for 24 h at 4 °C and then washed twice with 1 × PBS. Decalcification was carried out overnight in 10% w/w EDTA (pH 8.0) after which the remaining tissue was washed to remove residual EDTA. All solutions contained 0.65 mol l−1 sucrose to ensure minimal osmotic stress. Tissue from decalcified foraminifera was embedded in paraffin wax using an enclosed automated tissue processor (Shandon Excelsior ES, Thermo Fisher Scientific Inc., Waltham, MA, USA) and a standard ethanol and xylen dehydration method. The embedded foraminifera were cut into 15 μm sections using a microtome and dried onto hydrophilic slides (StarFrost, Waldemar Knittel, Braunschweig, Germany). Tissue sections were visualised on an inverted fluorescence microscope (Eclipse-Ti, Nikon Corporation, Tokyo, Japan) using the auto-fluorescence of the animal tissue (FITC, blue/green ex 475–490 nm/em 500–540 nm) and symbiont chlorophyll (TexasRed, green/red ex 532–587 nm/em 595 nm).
Video analysis of symbiont movement
Using an inverted fluorescence microscope symbionts were imaged inside the chambers of live foraminifera in the presence and absence of cytochalasin B (n=18). Chambers of the foraminifera were imaged in two fluorescent wavelengths (Green—FITC, red—TexasRed) at a total of × 400 magnification, utilising the auto-fluorescence of the skeleton and symbionts, respectively. Foraminifera were left in the dark and an image taken every minute for 15 min. For analyses, only chambers which were less than half full of symbionts were included to avoid bias resulting from clumping of cells, and only cells visible for the full length of the image series were included in the analyses. The movement of individual symbionts was measured by calculating the change in location of each symbiont between images.
Statistical analysis
Chlorophyll a fluorescence and relative change in pixel intensity as a function of time were analysed using repeated measures analysis of variance for the interactive terms of treatment and time (α=0.05). Differences in photoprotective pigments, symbiont movement in the presence and absence of cytochalasin B, as well as the relative change in reflectance and effective quantum yield of PSII in the presence and absence of DCMU were analysed using one-way analysis of variance (α=0.05). All data were checked a priori for normality and homoscedasticity. In the cases where data failed to meet the assumptions, data were transformed. All data were analysed using statistical software package SPSS (v.22; IBM, Armonk, NY, USA).
Results
Symbionts exhibit negative phototaxis under photosynthetic stress
Exposure to incremental increases in irradiance resulted in a significant decline (P=0.001) in the effective quantum yield of PSII (ΔF/FM'). The ΔF/FM' dropped to 0.1 when exposed to 800 μmol photons m−2 s−1 for 1 h (Figure 1a). The recovery of ΔF/FM' in the foraminifera exposed to the high-light treatment reached 75% of the initial ΔF/FM' whereas there was no change in ΔF/FM' in the foraminifera maintained at 130 μmol photons m−2 s−1 (Figure 1a). In both of the light treatments, the underside of M. vertebralis showed significantly higher (P<0.001) quantum yield values (0.590; dark-adapted) and no change over time (Figure 1a), suggesting the cells located on the underside of the test were completely protected from the high irradiances. Excitation pressure over PSII (QM), a measure of the proportion of open PSII reaction centres, increased significantly with increasing irradiance (P<0.001), reaching a maximum value of 0.83 at the highest irradiance (Figure 1b). Consistent with the recovery in ΔF/FM', there was a reversal of QM to values similar to those measured at 200 μmol photons m−2 s−1 (T2) after 1 h in recovery light (P=0.001). Dark-adapted maximum quantum yield values (FV/FM) did not change from before to after the experiment, with initial FV/FM values of 0.544±0.020 and 0.549±0.008, and recovered FV/FM values of 0.562±0.013 and 0.561±0.017 in the control and light-treated foraminifera (n=4), respectively. The de-epoxidation ratio of the photoprotective xanthophyll pigments increased to a maximum at 400 and 800 μmol photons m−2 s−1 (P<0.001) and recovered to values measured at 200 μmol photons m−2 s−1 (T2) an hour after being returned to control light levels (130 μmol photons m−2 s−1; Figure 1b), following the same pattern as QM. The xanthophyll de-epoxidation ratio did not change in M. vertebralis under constant light.
Change in photophysiology and reflectance under different irradiance treatments over time. (a) Effective quantum yield of PSII (ΔF/FM') for the surface (circles) and underside (triangles) of Marginopora vertebralis exposed to constant light (CL; black) and increasing light (IL; blue) over 3 h (200, 400 and 800 μmol photons m−2 s−1) with a final hour of recovery (130 μmol photons m−2 s−1) (n=8). (b) Excitation pressure over PSII (QM) on the surface of M. vertebralis (circles; n=8) and the de-epoxidation ratio of photoprotective pigments (bars; n=3) exposed to constant low (130 μmol photons m−2 s−1) light (black) and increasing irradiance over 3 h+recovery (blue). (c) Relative change in average pixel intensity on the surface (circles) and underside (triangles) of M. vertebralis exposed to constant (black) and increasing light over 3 h+recovery (blue) (n=5–8). (d) Spectral reflectance as a percentage of a pure white standard measured on the surface of M. vertebralis exposed to increasing irradiances. Arrows indicate characteristic absorption wavelengths of Symbiodinium: chlorophyll a (435–440, 675 nm), chlorophyll c (460 nm) and peridinin (480–490 nm), dashed lines indicate s.e.m. (n=8). (e) Total integrated reflectance at the surface of M. vertebralis exposed to increasing irradiances over time (T0–T3) (n=8). (f) Photographs illustrating the sequential whitening (from top left to bottom right) of one M. vertebralis exposed to high light. Scale bar=5 mm. Data represent mean±s.e.m. Asterisk (*) indicates values that are significantly different between light treatments and superscript letters denote significantly different over time (P<0.05). A full colour version of this figure is available at the ISME journal online.
Light stress results in symbiont retraction into the test
Symbiont retraction into the test was measured via changes in surface colour and reflectance. As the symbionts withdrew, more of the white test was exposed, causing an increase in the relative pixel intensity (whiteness) of the corresponding image or increasing spectral reflectance. The downward migration of symbionts, as measured by change in surface colour (Supplementary Movie 1), resulted in a significant increase in pixel intensity (whitening due to exposure of the calcite test and loss of absorption by symbionts) with increased irradiance (P=0.002; blue circles). There was no change in pixel intensity of the control foraminifera on either their exposed surface (black circles) or shaded underside (black triangles; Figure 1c). There was, however, a decrease (P=0.002) in pixel intensity (darkening) on the underside of the light-treated foraminifera (blue triangles; Figure 1c), indicative of an increase in symbiont density on the shaded side. An increase (P<0.001) in absolute reflectance (relative to a white Spectralon standard) was detected with increased irradiance (Figure 1d), with the total integrated reflectance increasing from 30% in foraminifera under initial light conditions to 50% reflectance after exposure for 1 h at 800 μmol photons m−2 s−1 (Figure 1e; P<0.001). The change in total reflectance was uniform across all wavelengths, where the major absorption bands of the chlorophyll a, c2 and peridinin of the Symbiodinium changed equally with retraction into the test (Figures 1d and f), suggesting no change in relative composition or loss of pigments.
To confirm a vertical downward migration of symbionts through the interstitial channels of the foraminiferal skeletal structure and visualise the localisation of symbionts within the test, tissue sections were made of foraminifera taken from low (130 μmol photons m−2 s−1), moderate (400 μmol photons m−2 s−1) and high light (800 μmol photons m−2 s−1) treatments (Figure 2). Histological examination demonstrated that the symbionts relocated to the far side of the foraminiferal test when incoming irradiance was sufficiently high (Figure 2b). Although the animal tissue fluoresced both in red and green, the stronger red auto-fluorescence of the algal chlorophyll resulted in a clear red colouration where symbionts were present in the tissue. After 1 h under control light, the majority of the symbionts were close to the surface of the foraminifera (Figure 2b, left). At moderate irradiance (400 μmol photons m−2 s−1), the symbionts were distributed throughout the test (Figure 2b, middle), whereas at the highest irradiance (800 μmol photons m−2 s−1) the greatest symbiont density was seen in the under-most chambers of the foraminifera (Figure 2b, right).
Tissue sections illustrating the localisation of the symbionts within the test of Marginopora vertebralis exposed to 130, 400 and 800 μmol photons m−2 s−1. (a) Complete tissue section of an M. vertebralis tests, scale bar=200 μm, (b) close up of three different tissue sections from foraminifera exposed to different light intensities (indicated in the picture), scale bar=50 μm. Green is the auto-fluorescence of the animal tissue and red is the symbiont chlorophyll. A full colour version of this figure is available at the ISME journal online.
Symbiont migration is host-mediated
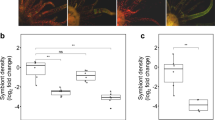
The symbiont morphology was coccoid (Supplementary Figure S2), indicative of non-motile cells (Freudenthal, 1962). Cells incubated with a fluorescent membrane vacuole stain (Trautman et al., 2002) were highly fluorescent, showing the presence of a symbiosome encasing each cell (Supplementary Figure S2). To further investigate the host mechanism of symbiont relocation, we measured symbiont movement in the presence of the actin filament inhibitor cytochalasin B. The reduction in the photosynthetic efficiency (ΔF/FM') of foraminifera exposed to high light, was greater in those treated with cytochalasin B (5 μg ml−1; Figure 3a; P=0.001), where the ΔF/FM' at 800 μmol photons m−2 s−1 was zero (indicative of symbiont death) in six of the eight specimens, highlighting the efficacy of symbiont retraction in providing photoprotection. The addition of cytochalisin B resulted in a 70% reduction in symbiont retraction at 800 μmol photons m−2 s−1 compared with the controls (Figure 3b; P<0.001). Similarly, time lapse fluorescence microscopy of individual test chambers (Figure 4a) showed a significant decline in symbiont movement in the presence of the actin filament inhibitor (Supplementary Movie 2), where the addition of 20 μg ml−1 resulted in a 90% reduction in movement (Figure 4b; P<0.001). Importantly, cytochalasin B has been shown not to affect movement in ciliates or flagellates at concentrations up to 50 μg ml−1 (Carter, 1967), more than twice the concentration employed in this study (5–20 μg ml−1). As such, it is unlikely that the cytochalasin B would have inhibited any movement driven by Symbiodinium.
Change in photosynthetic efficiency and pixel intensity in the presence of the actin inhibitor cytochalasin B. (a) Effective quantum yield of PSII (ΔF/FM') at constant (CL; black) and increasing (IL; blue) light intensities in the presence (cyto; circles) and absence (DMSO; triangles) of cytochalasin B. Insert shows the ΔF/FM' at 800 μmol photons m−2 s−1 as a percentage of the initial values. (b) Relative change in pixel intensity at constant (black) and increasing light (blue) intensities in the presence (circles) and absence (triangles) of cytochalasin B. Data represent mean±s.e.m., n=6–8. Asterisk (*) indicates values that are significantly different between light treatments and superscript letters denote significantly different over time (P<0.05). A full colour version of this figure is available at the ISME journal online.
Change in symbiont motility in the presence of cytochalasin B. (a) S ymbiodinium (red) within individual chambers of Marginopora vertebralis test (green), (b) average speed of movement of S ymbiodinium within chambers incubated with 0, 10 and 20 μg ml−1 of cytochalasin B, respectively, as a percent of control (data were square root transformed; n=18). Scale bar=25 μm. Data represent mean±s.e.m. Superscript letters denote significant difference between treatments (P<0.05). A full colour version of this figure is available at the ISME journal online.
Symbiont photosynthetic activity drives stress signalling to the host
To explore whether the signal for symbiont retraction was directly related to photosynthetic stress, we sought to find changes in the symbiont response when photosynthesis was reduced. We used the photosynthetic inhibitor DCMU with the expectation that failure to remove the symbionts under high light would indicate communication between the symbiotic partners is photosynthetically-driven. In the DMSO control treatment, we measured a 73% reduction in photosynthetic efficiency at high light compared with the controls (Figure 5; P<0.001), where effective quantum yield of PSII (ΔF/FM') in the control was 0.45 at 130 μmol photons m−2 s−1 and dropped to around 0.12 at 800 μmol photons m−2 s−1, equivalent to the ΔF/FM' in the first set of experiments (Figure 1a). In contrast, in the presence of DCMU, ΔF/FM' was 0.14 at control irradiances dropping below 0.05 at higher light (Figure 5), where six out of the eight foraminifera had no variable fluorescence. We found that DCMU (10 μm) increased pixel intensity (symbiont retraction) by 20% and 27% in both the control (130 μmol photons m−2 s−1) and high-light (800 μmol photons m−2 s−1) treatments, respectively (Figure 5; P=0.001), showing there was an initial retraction (increased reflectance) of symbionts with the addition of DCMU. There was, however, no additional retraction with exposure to high light resulting in a 58% reduction in pixel intensity compared with control incubations (Figure 5).
Symbiont photosynthesis and vertical migration in the presence of DCMU. Relative change in pixel intensity (bars) and effective quantum yield of PSII (diamonds) in Marginopora vertebralis exposed to 130 μmol photons m−2 s−1 (black bars) and 800 μmol photons m−2 s−1 (blue bars) in the presence of DMSO (control) or DCMU (n=6–8). Error bars on ΔF/FM' are smaller than the symbol. Data represent the mean±s.e.m. Superscript letters denote significant difference between treatments (P<0.05). A full colour version of this figure is available at the ISME journal online.
Discussion
Photoprotection is essential in shallow reef systems where irradiance often exceeds the capacity for photosynthesis (Brown et al., 1999). Therefore, to avoid cellular damage or a breakdown in the symbiosis, symbiotic partnerships are dependent on the ability of the symbiont and/or host to regulate incoming irradiance. In the present study, the photophysiological responses of the symbionts within M. vertebralis were consistent with general phototrophic responses to high light, with a decline in photosynthetic efficiency and increase in energy dissipation, all indicative of light stress (Müller et al., 2001). However, the rapid recovery in photosynthetic efficiency and concomitant reversal of excitation pressure over PSII (QM) after return to low light suggests that long-term damage to the photosystem was largely avoided (Müller et al., 2001). In addition to increasing photosynthetic stress, we also showed that the symbionts relocated from the surface to the middle or underside of the foraminiferal test depending on the level of irradiance. These results demonstrate a correlation between the level of photosynthetic stress (light intensity) and the level of retraction, and thus protection. Although Symbiodinium showed photosynthetic plasticity, it is evident that the physical relocation of surface symbionts into the foraminiferal test contributed to preventing long-term photosynthetic damage. This is supported by a previous study which found that only 30% of the incoming irradiance was able to penetrate to the bottom of the test of M. vertebralis (Kohler-Rink and Kühl, 2000). In addition to the inherent shading effect of the test itself (Kohler-Rink and Kühl, 2000), the increased reflectivity of the test decreased the incoming irradiance by an additional 20% upon symbiont retraction. The efficacy of this photoprotective strategy is supported by the high photosynthetic activity measured in symbionts on the underside of the test during exposure to high irradiance and the rapid reversibility in photosynthetic quenching, QM and xanthophyll pigment epoxidation of surface symbionts when incoming irradiance was lowered. Further support for the effectiveness of the protection offered by the host is provided by the fluorescence measurements in the presence of cytochalasin B, which showed that when vertical migration was prevented, the photosynthetic activity of the symbionts exposed to high light was severely inhibited with no variable fluorescence detectable in six of the eight specimens. It cannot be ruled out, however, that this effect might also have been a result of some inhibitory effect of the cytochalasin B on the chloroplast repair system in the symbionts. The small yet significant increase in pixel intensity (whitening) observed at the highest light level in the presence of cytochalasin B could in fact be attributed to loss of colouration from photobleaching of the chlorophyll in the immobilised symbionts. If so, this further supports the importance of this mechanism in the photoprotection of the symbionts in M. vertebralis.
The concept of phototaxis as a means for optimising light for photosynthesis in free-living microalgae is well studied. However, due to the inherent complexity of organisms living in symbioses, less is known about light regulation in symbiotic algae, and much less about the role of the host in this regulation. Until now, the only research on phototaxis in benthic endosymbiotic foraminifera has been focused on their propensity for seeking out shade through pseudopodal locomotion when exposed to high irradiances (Sinutok et al., 2013; Zmiri et al., 1974; Lee et al., 1980). In the only other study reporting the observation of intracellular phototaxis in M. vertebralis, the movement was believed to be driven by the symbionts themselves through flagella propulsion (Ross, 1972). However, the data presented here provides strong evidence for the phototaxic movement being host- rather than symbiont-driven: the coccoid, as opposed to gymnodinioid morphology of the symbionts is indicative of Symbiodinium in their non-motile, vegetative stage (Freudenthal, 1962), and the presence of a symbiosome membrane around the symbiont cells preclude the likelihood that symbionts could propel or move themselves within the host tissue. We saw a significant reduction in symbiont retraction and symbiont movement within individual chambers of the foraminifera test when actin filament contraction was inhibited (Estensen et al., 1971). This corroborates that the movement is host-mediated as well as provides the first insight into the mechanisms behind this movement.
The ability to adjust intracellular symbiont position is likely an important means to optimise carbon production, calcification and minimise photosynthetic damage, and can be described as akin to the chloroplastic migration observed in phototrophic organisms, also known as chloroplast photorelocation (Suetsugu and Wada, 2012). This light-dependent process optimises photosynthesis and photoprotection through dispersion or aggregation of the chloroplasts to maximise light capture or shading, respectively (Wada, 2013). The action of chloroplast photorelocation is driven by the common motor proteins actin and myosin (Suetsugu and Wada, 2012), which together with microtubules are responsible for the movement of cellular organelles in eukaryotic organisms. In the case of photosymbiotic organisms, however, the chloroplast is replaced by an entire algal cell. One of few known examples of photorelocation in a symbiotic organism is that of the single-celled protist Paramecium bursaria. Known as the ‘green Paramecium’, P. bursaria is symbiotic with the green, non-motile microalgae Chlorella. When exposed to high light, P. bursaria will aggregate its symbionts, presumably to shade both the host and the Chlorella cells, whereas it distributes the Chlorella cells evenly in low light, maximising light uptake (Summerer et al., 2009). The phototaxis shown here demonstrates photorelocation in M. vertebralis as a means of optimising light capture and protection. The dynamic nature of the regulation of endosymbiont location by the host suggests that it is closely coupled with the intensity of the incoming irradiance and the time of exposure. Furthermore, the ability for M. vertebralis to move its symbionts within its test may explain its propensity to attach to opaque surfaces (Sinutok et al., 2011; Sinutok et al., 2013), thereby eliminating light input from the attached side and thus optimise the efficacy of shading and photoprotection during the retraction of the symbionts.
The vertical migration away from high light, demonstrates a link between the symbiont stress and the host’s regulation of symbiont positioning, indicative of direct communication between the two partners. In high light, the photosynthetic stress experienced by the symbiont is converted to a signal that leads to reorganisation by the protist to ensure no damage to its energy-producing ‘solar cells’. This not only reduces the likelihood of photosynthetic damage from increased reactive oxygen, but enables carbon fixation and possibly light-dependent calcification, as observed in other foraminifera (Hallock, 1981; Lea et al., 1995), to continue unimpeded. By chemically reducing the photosynthetic efficiency of the symbionts (addition of DCMU), under control light conditions, partial retraction of symbionts was observed, indicative of photosynthetic stress. However, the addition of high light further quenched the photosystem to dysfunctional levels (ΔF/FM'<0.05) but did not induce any further vertical migration. The lack of movement under high light indicates a photosynthetically derived communication signal between partners, where the host’s removal of its symbionts relies on an infochemical or signal that is generated by photosynthesis. Furthermore, as DCMU blocks the transport of electrons through the photosynthetic electron transport chain at the beginning of the photochemical pathway, it would suggest that any signalling molecule is a result of downstream processes, relying on photosynthates (ATP, NADPH) derived from photosynthetic electron transport and carbon fixation. One potential candidate signal molecule worthy of investigation could be a type of reactive oxygen that is produced during photosynthetic stress (Lesser 2006).
This study has described negative phototaxis of symbionts in M. vertebralis in response to high light, and confirmed that this movement is not flagellate driven. We uncovered a novel mechanism for host-mediated photoprotection via the intracellular relocation of endosymbionts, whereby the host, upon receiving a signal from the symbionts, mobilises cellular proteins to relocate the symbionts deeper within its calcium carbonate test, thus providing protection and ensuring the health of the partnership. Furthermore, the behavioural response described here suggest phototaxis is driven by symbiont stress signalling, where the infochemical is derived from downstream processes of the photosynthetic electron transport chain. Our findings highlight the central importance of the host in photosymbiotic photoprotection. The dynamic nature of the photoregulatory response described here opens up new avenues to investigate symbiont–host stress physiology and symbiont–host signalling for other photosymbiotic species, such as corals, where the largest knowledge gap is the communication or signalling between the host and the symbiont during physiological stress that results in coral bleaching, the catastrophic collapse of the symbiotic partnership.
References
Baker AC . (2003). Flexibility and specificity in coral-algal symbioses: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Syst 34: 661–689.
Brown BE, Ambarsari I, Warner ME, Fitt WK, Dunne RP, Gibb SW et al. (1999). Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18: 99–105.
Brown BE, Downs CA, Dunne RP, Gibb SW . (2002). Preliminary evidence for tissue retraction as a factor in photoprotection of corals incapable of xanthophyll cycling. J Exp Mar Biol Ecol 277: 129–144.
Carter SB . (1967). Effects of cytochalasin on mammalian cells. Nature 213: 261–264.
Dimond JL, Holzman BJ, Bingham BL . (2012). Thicker host tissues moderate light stress in a cnidarian endosymbiont. J Exp Biol 215: 2247–2254.
Doo SS, Hamylton S, Byrne M . (2012). Reef-scale assessment of intertidal large benthic foraminifera populations on one tree island, great barrier reef and their future carbonate production potential in a warming ocean. Zool Stud 51: 1298–1307.
Dove SG, Lovell C, Fine M, Deckenback J, Hoegh-Guldberg O, Iglesias-Prieto R et al. (2008). Host pigments: potential facilitators of photosynthesis in coral symbioses. Plant Cell Environ 31: 1523–1533.
Estensen RD, Rosenberg M, Sheridan JD . (1971). Cytochalasin B: microfilaments and contractile processes. Science 173: 356–358.
Freudenthal HD . (1962). Symbiodinium gen nov and Symbiodinium microadriaticum sp nov, a zooxanthella—taxonomy, life cycle, and morphology. J Protozool 9: 45–52.
Hallock P . (1981). Light dependence in Amphistegina. J Foramin Res 11: 40–46.
Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE . (2004). Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc Lond B 271: 1757–1763.
Iglesias-Prieto R, Trench RK . (1994). Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux-density. Mar Ecol Prog Ser 113: 163–175.
Iglesias-Prieto R, Trench RK . (1997). Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar Biol 130: 23–33.
Jones RJ, Hoegh-Guldberg O . (2001). Diurnal changes in photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ 24: 89–99.
Khare N, Nigam R . (2000). Laboratory experiment to record rate of movement of cultured benthic foraminifera. ONGC Bull 37: 53–61.
Kohler-Rink S, Kühl M . (2000). Microsensor Studies of photosynthesis and respiration in larger symbiotic foraminifera. I. The physico-chemical microenvironment of Marginopora vertebralis Amphistegina lobifera and Amphisorus hemprichii. Mar Biol 137: 473–486.
Langer MR, Silk MT, Lipps JH . (1997). Global ocean carbonate and carbon dioxide production; the role of reef foraminifera. J Foramin Res 27: 271–277.
Lea DW, Martin PA, Chan DA, Spero HJ . (1995). Calcium uptake and calcification rate in the planktonic foraminefera orbulina universa. J Foramin Res 25: 14–23.
Lee JJ, McEnery ME, Garrison JR . (1980). Experimental studies of larger foraminifera and their symbionts from the Gulf of Eilat on the Red Sea. J Foramin Res 10: 31–47.
Lee JJ . (2006). Algal symbiosis in larger foraminifera. Symbiosis 42: 63–75.
Lesser MP . (2006). Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68: 253–278.
Müller P, Li X-P, Niyogi KK . (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566.
Pawlowski J, Holzmann M, Fahrni JF, Pochon X, Lee JJ . (2001). Molecular identification of algal endosymbionts in large miliolid foraminifera: 2. Dinoflagellates. J Euk Microbiol 48: 368–373.
Ross CA . (1972). Biology and ecology of Marginopora vertebralis (Foraminiferida), Great Barrier Reef. J Protozool 19: 181–192.
Salih A, Larkum ADW, Cox G, Kühl M, Hoegh-Guldberg O . (2000). Fluorescent pigments in corals are photoprotective. Nature 408: 850–853.
Schneider CA, Rasband WS, Eliceiri KW . (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675.
Schreiber U . (2004) Pulse-amplitude-modulated (PAM) fluorometry and saturation pulse method. In: Papagiorgiou GG (ed). Advances in Photosynthesis and Respiration. vol 19. Springer: Dordrecht, Netherlands, pp 279–319.
Sinutok S, Hill R, Doblin MA, Wuhrer R, Ralph PJ . (2011). Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol Oceanogr 56: 1200–1212.
Sinutok S, Hill R, Doblin MA, Ralph PJ . (2013). Diurnal photosynthetic response of the motile symbiotic benthic foraminiferan Marginopora vertebralis. Mar Ecol Prog Ser 478: 127–138.
Suetsugu N, Wada M . (2012) Chloroplast photorelocation movement: a sophisticated strategy for chloroplasts to perform efficient photosynthesis. In: Najafpour MM (ed). Advances in Photosynthesis—Fundamental Aspects InTech, pp 215–234.
Summerer M, Sonntag B, Hörtnagl P, Sommaruga R . (2009). Symbiotic ciliates receive protection against UV damage from their algae: a test with paramecium bursaria and chlorella. Protist 160: 233–243.
Trautman DA, Hinde R, Cole L, Grant A, Quinnell R . (2002). Visualisation of the symbiosome membrane surrounding cnidarian algal cells. Symbiosis 32: 133–145.
van Heukelem L, Thomas C . (2001). Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J Chromatogr A 910: 31–49.
Wada M . (2013). Chloroplast movement. Plant Sci 210: 177–182.
Wangpraseurt D, Larkum AWD, Franklin J, Szabo M, Ralph PJ, Kühl M . (2014). Lateral light transfer ensures efficient resource distribution in symbiont-bearing corals. J Exp Biol 217: 489–498.
Zmiri A, Kahan D, Hochstein S, Reiss Z . (1974). Phototaxis and thermotaxis in some species of Amphistegine (Foraminifera). J Protozool 21: 133–138.
Acknowledgements
We are grateful for the technical assistance of Jacqueline Loyola-Echeverria with the histological analysis and thank Dr Jean-Baptiste Raina for his helpful comments on the manuscript. We would also like to thank the staff at the Heron Island research station (HIRS). KP was supported by a UTS Chancellor’s Postdoctoral Fellowship. Marginopora vertebralis were collected under the Great Barrier Reef Marine Parks permit G14/36977.1 issued to KP and DAN.
Author Contributions
KP and DAN designed and performed research; PJR contributed analytical tools; KP and DAN analysed the data; KP and DAN wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Petrou, K., Ralph, P. & Nielsen, D. A novel mechanism for host-mediated photoprotection in endosymbiotic foraminifera. ISME J 11, 453–462 (2017). https://doi.org/10.1038/ismej.2016.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2016.128
This article is cited by
-
Taming the perils of photosynthesis by eukaryotes: constraints on endosymbiotic evolution in aquatic ecosystems
Communications Biology (2023)
-
Kleptoplast distribution, photosynthetic efficiency and sequestration mechanisms in intertidal benthic foraminifera
The ISME Journal (2022)
-
Heavy metal incorporation in foraminiferal calcite under variable environmental and acute level seawater pollution: multi-element culture experiments for Amphisorus hemprichii
Environmental Science and Pollution Research (2022)
-
Disentangling thermal stress responses in a reef-calcifier and its photosymbionts by shotgun proteomics
Scientific Reports (2018)