Abstract
Evidence for anaerobic ammonium oxidation in a paddy field was obtained in Southern China using an isotope-pairing technique, quantitative PCR assays and 16S rRNA gene clone libraries, along with nutrient profiles of soil cores. A paddy field with a high load of slurry manure as fertilizer was selected for this study and was shown to contain a high amount of ammonium (6.2–178.8 mg kg−1). The anaerobic oxidation of ammonium (anammox) rates in this paddy soil ranged between 0.5 and 2.9 nmolN per gram of soil per hour in different depths of the soil core, and the specific cellular anammox activity observed in batch tests ranged from 2.9 to 21 fmol per cell per day. Anammox contributed 4–37% to soil N2 production, the remainder being due to denitrification. The 16S rRNA gene sequences of surface soil were closely related to the anammox bacteria ‘Kuenenia’, ‘Anammoxoglobus’ and ‘Jettenia’. Most of the anammox 16S rRNA genes retrieved from the deeper soil were affiliated to ‘Brocadia’. The retrieval of mainly bacterial amoA sequences in the upper part of the paddy soil indicated that nitrifying bacteria may be the major source of nitrite for anammox bacteria in the cultivated horizon. In the deeper oxygen-limited parts, only archaeal amoA sequences were found, indicating that archaea may produce nitrite in this part of the soil. It is estimated that a total loss of 76 g N m−2 per year is linked to anammox in the paddy field.
Similar content being viewed by others
Introduction
For decades, ‘heterotrophic denitrification’ was the only known pathway for nitrogen loss to the atmosphere. The discovery of anaerobic oxidation of ammonium (anammox) coupled to nitrite reduction with N2 as the end product in natural ecosystems challenged this view (Thamdrup and Dalsgaard, 2002; Dalsgaard et al., 2003; Kuypers et al., 2003).
The anammox process is mediated by bacteria affiliated to order the Brocadiales, which are part of the phylum Planctomycetes (Jetten et al., 2010). At present, five genera of anammox bacteria have been described, ‘Brocadia’, ‘Kuenenia’, ‘Anammoxoglobus’, ‘Jettenia’ and ‘Scalindua’. In addition to biodiversity, much of the research into anammox in natural environments was focused on its role in the oceanic nitrogen cycle. Anammox activity and biomarkers are found in many marine ecosystems, including continental margin sediments (Engström et al., 2005; Trimmer and Nicholls, 2009), estuarine sediments (Risgaard-Petersen et al., 2004; Rich et al., 2008) and anoxic marine waters (Kuypers et al., 2003, 2005). Presently, up to 67% of N2 production in marine sediments may be attributed to the anammox process (Dalsgaard et al., 2005). However, few studies have addressed the anammox process in freshwater wetland ecosystems (Zhang et al., 2007; Fan et al., 2010; Humbert et al., 2010), and until now there has been no report on anammox activity or biodiversity in paddy fields, which are one of the most significant nitrogen sinks in terrestrial ecosystems (Kögel-Knabner et al., 2010).
Rice is the globally most important food grown on almost 155 million hectare of the Earth’s surface for more than 50% of the world population. With the rapid increase of the application of various nitrogen fertilizers, the rice grain yield has also increased substantially over the last decades (Nicolaisen et al., 2004). However, use of large amounts of fertilizer has also resulted in large amounts of nitrogen loss through NH3 volatilization, N2O emission and leaching (Xing and Zhu, 2000). Whether anammox bacteria exist and have a role in paddy field nitrogen cycling is unknown at the moment.
Hence, the aim of the present study was to investigate the activity, biodiversity, and qualitative and quantitative importance of anammox bacteria in the nitrogen cycle of a selected paddy field in Southern China.
Experimental procedures
Soil samples and background
In many anammox bioreactor studies, a high oxidant ammonia concentration was reported to stimulate anammox bacteria (Kartal et al., 2008). Therefore, a paddy field with long-term fertilization was selected for this study. The sampling site is located close to Jiaxing city (Zhejiang Province, China; E120°41′54.7′′ N30°45′51.4′′) and represents a typical agricultural region of subtropical Southern China. It has a subtropical monsoon climate with an annual rainfall of 1300 mm and annual average temperature of 18 °C. The soil has been planted with a rice/wheat rotation and supplied with a high load of slurry manure containing substantial amounts of ammonium (Supplementary Table S1) for more than 25 years. The feeding frequency of the slurry application is about two times per month from April to October and one time per month from November to March. The total rate of ammonia fertilization is about 320 g N m−2 per year. Five soil cores (5-cm diameter and 100-cm depth) were collected from the plot in November 2008. The soil cores were placed in sterile plastic bags, sealed and transported to the laboratory on ice. Later, they were sliced every 10 cm and mixed at every depth to form one composite sample. One part was incubated to determine nitrification and anammox activities immediately after arrival, and another part was sieved through 2.0 mm for analysis of chemical components, and subsamples were stored at −80 °C for later DNA extraction and molecular analysis.
Chemical analytical procedures of soils
Ammonium, nitrite+nitrate were extracted from the soil with 2 M KCl and measured using a Continuous Flow Analyzer (SAN plus, Skalar Analytical B.V., Breda, the Netherlands). The total nitrogen, total phosphorus and Mn (II–IV) content of the soil samples was also measured according to standard methods (Bao, 2000). Soil pH was determined after mixing with water at a ratio (soil/water) of 1:2.5, and soil organic matter was determined by K2Cr2O7 oxidation method. All analyses were performed on triplicate soil samples. The oxygen concentration in fresh soil was measured using OXY Meter S/N 4164 with stainless electrode sensor (Unisense, Aarhus, Denmark), according to Gundersen et al. (1998).
Measuring anammox and denitrification rate with 15N-labeled ammonium and nitrate
The presence, activity and potential of anammox and denitrifying bacteria were measured as described in Risgaard-Petersen et al. (2004) and Engström et al. (2005). Soil samples of known weight and density were transferred to the He-flushed, 6.6-ml glass vials (Exetainer, Labco, High Wycombe, Buckinghamshire, UK), together with N2-purged media water from the paddy field. The resulting soil slurries were then preincubated for 24 h to remove residual NOx− and oxygen. Subsequently, 100 μl of N2-purged stock solution of each isotopic mixture, that is, (1) 15NH4+ (15N at 99.6%), (2) 15NH4++14NO3− and (3) 15NO3− (15N at 99%) was injected through the septa of each vial, resulting in a final concentration of about 100μM N. Incubation of the slurries was stopped at hourly intervals by injecting 200 μl of a 7 M ZnCl2 solution. The rate and potential contribution to N2 formation of either anammox or denitrification were calculated from the produced 29N2 and 30N2, measured by continuous flow Isotope Ratio Mass Spectrometry (MAT253 with Gasbench II and autosampler (GC-PAL), Bremen, Thermo Electron Corporation, Finnigan, Germany) as described by Thamdrup and Dalsgaard (2002).
Measuring potential nitrification rate
Potential nitrification rates were measured using the chlorate inhibition method (Kurola et al., 2005). Briefly, 5.0 g of fresh soil was added to 50-ml centrifuge tubes containing 20 ml of phosphate buffer solution (NaCl, 8.0; KCl, 0.2; Na2HPO4, 0.2; NaH2PO4, 0.2g l−1; pH 7.4) with 1 mM (NH4)2SO4. Potassium chlorate was added to the tubes with a final concentration of 10 mM to inhibit nitrite oxidation. The suspension was incubated in the dark at 18 °C for 24 h; after that, nitrite was extracted with 5 ml of 2 M KCl and determined spectrophotometrically at 540 nm with N-(1-naphthyl) ethylenediamine dihydrochloride.
DNA isolation and PCR
DNA was extracted from 0.25 g of soil using the Fast DNA SPIN Kit for Soil (QBIOgene Inc., Carlsbad, CA, USA), with a beating time of 20s and a speed setting of 5.5 m. A nested-PCR assay was conducted to detect anammox 16S rRNA genes. The initial amplification was carried out using the PLA46f-630r primer combination with a thermal profile of 96 °C for 10 min, followed by 35 cycles of 60 s at 96 °C, 1 min at 56 °C, 1 min at 72 °C (Juretschko et al., 1998; Neef et al., 1998). After the first step, a 500-times diluted (1 μl) PCR product was used as template for the second amplification with Amx368f-Amx820r primers using a thermal profile of 96 °C for 10 min, followed by 25 cycles of 30 s at 96 °C, 1 min at 58 °C, 1 min at 72 °C (Schmid et al., 2005).
Quantitative PCR (qPCR) assay
Anammox bacterial abundance was quantified with the TaqMan fluorogenic PCR method developed by Hamersley et al. (2007). qPCR was performed with the primers AMX-808-F and AMX-1040-R, and the TaqMan probe AMX-931 under the same condition described by Hamersley et al. (2007) using an ABI 7500 Real Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Standard plasmid carrying anammox 16S rRNA genes was generated by cloning 16S rRNA genes from surface soil as described above. Standard curves were obtained with serial dilutions of 1 ng μl−1 of the plasmid DNA containing anammox bacterial 16S rRNA genes.
Cloning, sequencing and phylogenetic analysis
The purified PCR products were ligated and cloned using the pGEMT-easy (Promega, Madison, WI, USA). In all, 100 clones were picked for each of the PCR products. The inserts were analyzed with restriction endonucleases HaeIII and RsaI. The clones of representative digestion patterns were sequenced with an ABI PRISM 3730XL automated-sequencer (Biomed Co., Beijing, China). The sequences obtained in this study for anammox bacteria are available in the under Accession numbers GU083863–GU083952.
Results
Identification and quantification of anammox bacterial abundance in paddy soil cores
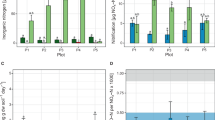
The vertical distribution profiles of ammonium, nitrite, nitrate, total nitrogen, total phosphorus, total organic matter, pH and oxygen in every 10 cm of the soil core are shown in Figure 1. The simultaneous decrease of both ammonium and nitrate with depth indicated the occurrence of denitrification, dissimilatory nitrate reduction to ammonium or nitrate reduction coupled to anammox.
To detect the presence of anammox bacteria, 16S rRNA genes from soil core samples were amplified every 10 cm, and a positive PCR product was obtained for all depth samples. The number of anammox 16S rRNA genes was then estimated from 8.6 × 105 to 1.1 × 107 copies per gram of soil with qPCR (Figure 2). The abundance of anammox cells in the surface soil was determined as 4.4 × 106 copies per gram of soil. The highest abundance was recorded in the soil horizon at 50–60 cm.
Biodiversity and community of anammox bacteria in surface and deep soil
To investigate the biodiversity of anammox bacteria in the paddy soil core, the 16S rRNA gene sequences retrieved from the clone libraries were analyzed using rarefaction analysis, Chao1 estimator and Shannon index calculations. A total of 11 operational taxonomic units (OTUs) (97% cutoff) was obtained from surface (four OTUs) and deep soil (seven OTUs) (Supplementary Figure S1). Phylogenetic analyses of anammox 16S rRNA bacterial sequences and related sequences deposited in GenBank showed the OTU #1 and OTU #2 in the surface soil were most closely affiliated to Anammoxoglobus and Jettenia, respectively (Figure 3). The other two OTUs in the surface soil were most closely related to Kuenenia (similarity 94.6–95.6%). A great change in the community structure of anammox bacteria was observed with soil depth. The seven OTUs in the deeper soil section all had a maximum similarity (95.4–96.6%) to Brocadia.
Evolutionary distance dendrograms constructed by neighbor-joining method using Kimura two-parameter distance with 1000 bootstrap in MEGA 4.0 package (Tamura et al., 2007) and showing the affiliations of the anammox sequences from surface and deep soil of Jiaxing paddy field. Designation of OTUs in bold includes the following information: number of sequences of surface (10–20 cm) and deep soil (90–100 cm) in the bracket parentheses. The detailed information of anammox clone sequences of each OTU and accession numbers in Genebank are listed in Supplementary Table S2.
Role and contribution of anammox bacteria in the paddy soil core
To determine anammox activity and the potential role of anammox as a N2 producer in the paddy soil, incubations were performed with homogenized soil under in situ temperature using a 15N-nitrogen isotope-pairing technique. The results showed that in the case of the slurries amended with 15NH4+ only, no significant accumulation of 15N2-labeled gas (29N2 and/or 30N2) could be observed at any depth of the soil core (Supplementary Figure S2A), indicating that all ambient 14NOx− had been consumed during the 24-h preincubations. When both 15NH4+ and 14NO3− were added, 29N2 accumulated at every soil depth with no accumulation of 30N2 (Supplementary Figure S2B). This pattern was reproducible, and the results showed that the anammox process was detectable in the soil core. In the case of the slurries amended with sole 15NO3− incubations, significant anammox and denitrification rates were observed.
The activity and contribution of anammox relative to denitrification was investigated (Supplementary Figure S2C). The results showed that anammox rates in this paddy soil (0–70 cm) ranged between 2.9 (0–10 cm) and 0.5 nmol N per gram of soil per hour (60–70 cm) (Figure 4). The anammox may contribute between 4% (50–60 cm) and 37% (30–40 cm) to N2 production at different depths of the paddy soil core. The cell-specific anammox rates were calculated using the qPCR data in Figure 2 and are shown in the right column of Figure 4. The specific activity ranged from 2.9 to 21 fmol per cell per day, which is well within the range of the reported values (2–20 fmol per cell per day; Strous et al., 1999a; Kuypers et al., 2003).
Quantitive analysis of various N cycle processes
The nitrification and denitrification rates were also determined to assess the potential source of nitrite for anammox in the paddy soil core (Figure 5a). The highest rate of nitrification (18 nmol N g−1 h−1) and denitrification (21 nmol N g−1 h−1) were both recorded in surface soil. Below the surface layer, denitrification decreased drastically to 3–10 nmol N g−1 h−1 and the same pattern was observed for nitrification, although the rates were higher (14–17 nmol N g−1 h−1) in the soil horizon at 0–40 cm. The results indicated that in the top soil, both partial denitrification and nitrification may provide anammox with nitrite, whereas in the deeper layers, mainly oxygen-limited nitrification may produce nitrite for anammox.
Vertical distribution of nitrification and denitrification activity (a) and the relevant archaeal and bacterial amoA gene copy numbers (b). The percentages of nitrification to nitrification + denitrification and ammonia-oxidizing bacteria to ammonia-oxidizing archaea are shown in the right-hand columns, respectively.
To get more detailed information on the potential of a combined nitrification–anammox process in the paddy soil core, qPCR assays on bacterial and archaeal amoA genes were performed according to the method in reference of Wang et al. (2011). The qPCR data showed that in the root zone, ammonia-oxidizing bacteria dominated the nitrification process in agreement with the nitrification rates, and the bacterial amoA gene numbers ranged from 8.8 × 105 to 3.2 × 108 g−1 dry soil (Figure 5b). After the root zone, the number of bacterial genes decreased drastically, and they could not be detected below 70 cm. However, the number of archaeal amoA copies were more or less constant with soil depth and the gene numbers ranged from 1.6 × 106 to 4.8 × 107 g−1 dry soil, resulting in an increase in the ratio of archaeal to bacterial amoA numbers, up to 10-fold in the deeper layers. This indicated that in the deeper paddy soil (50–100 cm) the nitrite needed for anammox may be produced by archaea.
Incubation of deep soil with elevated nitrate
In order to test the link between archaeal nitrification and anammox, the deeper soil was incubated with elevated nitrate concentrations (0.5 mM), according to the method in reference of Bartlett et al. (2008), and the archaeal amoA genes and anammox activity were monitored. The decrease in ammonium with time indicated that both nitrification and anammox occurred, although the number of arachaeal amoA genes did not change significantly (Figure 6). The 15N-test at the end of the 42-day incubation period showed that anammox activity had increased from 0.7 to 1.3 nmol N per gram of soil per hour.
Discussion
The discovery of anammox bacteria and activity in paddy fields alters our understanding of the mechanisms responsible for N loss from paddy fields. Our data indicate that a significant amount of N is lost via the anammox pathway. It was estimated that the ammonia loss attributed to anammox was 76 g N m−3 per year on the basis of soil density, porosity and rates obtained from slurry incubations. This indicates that about 23% of the applied ammonia fertilizers may be lost via the anammox process. Slurries incubations might, however, overestimate the in situ activity because substrates are supplied in excess. However, NH4+ and NOx are present at high concentrations in the upper 40 cm of the soil (Figure 1) and anammox contributed 46 g N per year (13% of the applied ammonia fertilizers) in this section. This significant loss of N due to anammox is similar to that for NH3 volatilization (up to 40%), leaching (9–15%), runoff (5–7%) and denitrification (up to 40%) (De Datta et al., 1991; Xing and Zhu, 2000; Zhao et al., 2009). Moreover, the cultivation practice of paddy fields provides favorable growth conditions for anammox bacteria (Zhu et al., 2010), including higher temperatures, water-logged oxygen-limiting conditions, long growth periods, and the coexistence of ammonia and nitrate.
The diversity of anammox bacteria has been studied in various ecosystems with molecular tools, and it has been shown that anammox bacteria exhibit apparently low biodiversity in marine ecosystems, where the diversity is restricted to the Scalindua genus only (Schmid et al., 2007). Also a low biodiversity has been observed in fresh water ecosystems (that is, Candidatus ‘Scalindua brodae’ in Lake Tanganyika (Schubert et al., 2006); Candidatus ‘B anammoxidans’ in river sediments (Zhang et al., 2007)). However, our findings are in agreement with recent studies in various soil ecosystems (Humbert et al., 2010; Hu et al., 2011) that have shown a high biodiversity in soil ecosystems, and in the present studies, no less than four genera of anammox bacteria, ‘Brocadia’, ‘Kuenenia’, ‘Anammoxoglobus’ and ‘Jettenia’ were detected. Apparently, in this paddy soil, many microniches exist that support the various ecophysiologies of the different anammox bacteria (Schmid et al., 2003; Kartal et al., 2007; Quan et al., 2008).
In addition to the biodiversity, the isotopic tracing experiments showed that the rate and contribution of anammox to N2 production were 0.5–2.9 nmol N per gram of soil per hour and 4–37% to N2 production, respectively. This is in the same range as other reported values in freshwater and estuarine environments (Risgaard-Petersen et al., 2004; Schubert et al., 2006; Erler et al., 2008; Dale et al., 2009; Hu et al., 2011). Moreover, the specific cell anammox rates (2.9–21 fmol per cell per day) were comparable to anammox rates found in bioreactors and marine water columns (2–20 fmol per cell per day; Strous et al., 1999a; Kuypers et al., 2003). The possible reasons for the substantial anammox rate and contribution in paddy fileds may be the high concentrations of ammonia introduced by slurry application manure. In these water-logged ecosystems, oxygen availabilty and production of nitrite and/or nitrate may be the controlling factors. Incubations with relatively high nitrate (0.5 mM) stimulated anammox activity in the deeper soil layers from 0.7 (0 days) to 1.3 nmol N per gram of soil per hour (42 days).
The potential interactions between particle-associated anammox bacteria and bacterial/archaeal partners in the present study have some similarity to those reported for marine snow particles (Lam et al., 2007, 2009; Woebken et al., 2007). In such oxygen-limited aggregates, cooperation between the ammonia-oxidizing prokaryotes can provide nitrite to anammox bacteria (Sliekers et al., 2002; Zhu et al., 2008; Yan et al., 2010). In this study, the nitrification rates in the deeper soil layers were always higher than the denitrification rates, indicating that partial nitrification processes might be the main source of nitrite for anammox, because 10 units of anammox need 10 units of nitrite and nitrification could supply 10, 12 or enough to sustain our measured rates. Kuypers et al. (2005) and Lam et al. (2007) also suggested that aerobic ammonium oxidation, rather than nitrate reduction, may be the source of nitrite for anammox in the Namibian OMZ and the Black Sea. The linking of archaeal and bacterial nitrification to anammox has also been demonstrated in marine systems, for example, the Black Sea (Lam et al., 2007) and Peruvian OMZ (Lam et al., 2009). The coexistence of ammonia-oxidizing archaea, ammonia-oxidizing bacteria and anammox has so far not been reported in terrestrial ecosystems.
Taken together, our results indicate that the anammox bacteria are present throughout the Jiaxing paddy field soil layer, and a loss of 76 g N m−2 per year linked to anammox was estimated for the paddy field, representing about 23% of the nitrogen fertilization rate. The possible link between archaeal and bacterial nitrification with anammox is now extended to terrestrial ecosystems. Future studies will need to address how tightly archaeal nitrification and anammox are coupled and if this interaction is also occurring and contributing to N loss in other oxygen-limited terrestrial ecosystems.
References
Bao SD . (ed.). (2000). Chemical Analysis for Agricultural Soil. China Agriculture Press: Beijing.
Bartlett R, Mortimer RJG, Morris K . (2008). Anoxic nitrification: evidence from humber Estuary sediments (UK). Chem Geol 250: 29–39.
Dalsgaard T, Canfield DE, Petersen J, Thamdrup B, Acũna-Gonzalez J . (2003). N2 production by the anammox reaction in the anoxic water of Golfo Dulce, Costa Rica. Nature 422: 606–608.
Dalsgaard T, Thamdrup B, Canfield DE . (2005). Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156: 457–464.
Dale OR, Tobias CR, Song B . (2009). Biogeographical distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in Cape Fear River Estuary. Environ Microbiol 11: 1194–1207.
De Datta SK, Buresh RJ, Samaon MI, Obcemea WN, Real JG . (1991). Direct measurement of ammonia and denitrification fluxes from urea applied to rice. Soil Sci Soc Am J 55: 543–548.
Engström P, Dalsgaard T, Hulth S, Aller RC . (2005). Anaerobic ammonium oxidation by nitrite (anammox): implications for N2 production in coastal marine sediments. Geochim Cosmochim Acta 69: 2057–2065.
Erler DV, Eyre BD, Davison L . (2008). The contribution of anammox and denitrification to sediment N2 production in a surface flow constructed wetland. Environ Sci Technol 42: 9144–9150.
Fan G, Zhu G, Wang Y, Wang C, Yin C . (2010). New functional microorganisms in nitrogen cycle restoration of river riparian ecosystems. Acta Scientiae Circumstantiae 30: 1558–1563.
Gundersen JK, Ramsing NB, Glud RN . (1998). Predicting the signal of O2 microsensors from physical dimensions, temperature, salinity, and O2 concentration. Limnol Oceanogr 43: 1932–1937.
Hamersley MR, Lavik G, Woebken D, Rattray JE, Lam P, Hopmans EC et al. (2007). Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnol Oceanogr 52: 923–933.
Humbert S, Tarnawski S, Fromin N, Mallet MP, Aragno M, Zopfi J . (2010). Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4: 450–454.
Hu BL, Rush D, van der Biezen E, Zheng P, van Mullekom M, Schouten S et al. (2011). A new anaerobic ammonium-oxidizing community enriched from peat soil. Appl Environ Microbiol 77: 966–971.
Jetten MSM, Op den Camp HJM, Kuenen JG, Strous M . (2010). Description of the order Brocadiales. In: Krieg NR, Staley JT, Hedlund BP, Paster BJ, Ward N, Ludwig W and Whitman WB (eds). Bergey's Manual of Systematic Bacteriology, vol. 4. Springer: Heidelberg, Germany. pp 506–603.
Juretschko S, Timmermann G, Schmid M, Schleifer KH, Pommerening-Röser A, Koops HP et al. (1998). Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol 64: 3042–3051.
Kartal B, Keltjens JT, Jetten MSM . (2008). Metabolism of Anammox. In: Encyclopedia of Life Sciences (ELS). John Wiley & Sons, Ltd: Chichester. pp 1–9, doi:10.1002/9780470015902.a0021315.
Kartal B, Rattray J, van Niftrik L, van de Vossenberg J, Schmid MC, Webb RI et al. (2007). Candidatus ‘Anammoxoglobus propionicus’ a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 30: 39–49.
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R et al. (2010). Biogeochemistry of paddy soils. Geoderma 157: 1–14.
Kurola J, Salkinoja-Salonen M, Aarnio T, Hultman J, Romantschuk M . (2005). Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiol Lett 250: 33–38.
Kuypers MMM, Lavik G, Wöbken D, Schmid M, Fuchs BM, Amann R . (2005). Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA 102: 6478–6483.
Kuypers MMM, Sliekers AO, Lavik G, Schmid M, Jorgensen BB, Kuenen JG et al. (2003). Anaerobic ammonium oxidation by anammox bacteria in Black Sea. Nature 422: 608–611.
Lam P, Jensen MM, Lavik G, McGinnis DF, Müller B, Schubert CJ et al. (2007). Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci USA 102: 6478–6483.
Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D et al. (2009). Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci USA 106: 4752–4757.
Neef A, Amann R, Schlesner H, Schleifer KH . (1998). Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144: 3257–3266.
Nicolaisen MH, Risgaard-Petersen N, Revsbech NP, Reichardt W, Ramsing NB . (2004). Nitrification–denitrification dynamics and community structure of ammonia oxidizing bacteria in a high yield irrigated Philippine rice field. FEMS Microbiol Ecol 49: 359–369.
Quan ZX, Rhee SK, Zuo JE, Yang Y, Bae JW, Park JR et al. (2008). Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ Microbiol 10: 3130–3139.
Rich JJ, Dale OR, Song B, Ward BB . (2008). Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments. Microb Ecol 55: 311–320.
Risgaard-Petersen N, Meyer RL, Schmidt M, Jetten MSM, Prast AE, Rysgaard S et al. (2004). Anaerobic ammonia oxidation in an estuarine sediment. Aquat Microb Ecol 36: 293–304.
Schmid MC, Maas B, Dapena A, de Pas-Schoonen KV, de Vossenberg JV, Kartal B et al. (2005). Biomarkers for in situ detection of anaerobic ammonium-oxidizing (anammox) bacteria. Appl Environ Microbiol 71: 1677–1684.
Schmid MC, Risgaard-Petersen N, van de Vossenberg J, Kuypers MMM, Lavik G, Petersen J et al. (2007). Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environ Microbiol 9: 1476–1484.
Schmid M, Walsh K, Webb R, Rijpstra WIC, van de Pas-Schoonen K, Verbruggen MJ et al. (2003). Candidatus Scalindua brodae, sp. nov Candidatus Scalindua wagneri, sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 26: 529–538.
Schubert CJ, Durisch-Kaiser E, Wehrli B, Thamdrup B, Lam P, Kuypers MMM . (2006). Anaerobic ammonium oxidation in a tropical freshwater system (Lake Tanganyika). Environ Microbiol 8: 1857–1863.
Sliekers AO, Derwort N, Gomez JLC, Strous M, Kuenen JG, Jetten MSM . (2002). Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res 36: 2475–2482.
Strous M, Fuerst JA, Kramer EH, Logemann S, Muyzer G, van de Pas-Schoonen KT et al. (1999a). Missing lithotroph identified as new planctomycete. Nature 400: 446–449.
Tamura K, Dudley J, Nei M, Kumar S . (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599.
Thamdrup B, Dalsgaard T . (2002). Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68: 1312–1318.
Trimmer M, Nicholls JC . (2009). Production of nitrogen gas via anammox and denitrification in intact sediment cores along a continental shelf to slope transect in the North Atlantic. Limnol Oceanogr 54: 577–589.
Zhang Y, Ruan XH, Op den Camp HJM, Smits TJM, Jetten MSM, Schmid MC . (2007). Diversity and abundance of aerobic and anaerobic ammonium-oxidizing bacteria in freshwater sediments of the Xinyi River (China). Environ Microbiol 9: 2375–2382.
Wang S, Wang Y, Feng X, Zhai L, Zhu G . (2011). Quantitative analyses of ammonia-oxidizing archaea and bacteria in the sediments of four nitrogen-rich wetlands in China. Appl Microbiol Biotechnol 90: 779–787.
Woebken D, Fuchs BM, Kuypers MMM, Amann R . (2007). Potential interactions of particle-associated anammox bacteria with bacterial and archaeal partners in the Namibian Upwelling System. Appl Environ Microbiol 73: 4648–4657.
Xing GX, Zhu ZL . (2000). An assessment of N loss from agricultural fields to the environment in China. Nutr Cycl Agroecosyst 57: 67–73.
Yan J, Op den Camp HJM, Jetten MSM, Hu YY, Haaijer SCM . (2010). Induced cooperation between marine nitrifiers and anaerobic ammonium-oxidizing bacteria by incremental exposure to oxygen. Syst Appl Microbiol 33: 407–415.
Zhao X, Xie Y, Xiong Z, Yan X, Xing G, Zhu Z . (2009). Nitrogen fate and environmental consequence in paddy soil under rice-wheat rotation in the Taihu lake region, China. Plant Soil 319: 225–234.
Zhu G, Jetten MSM, Kuschk P, Ettwig K, Yin C . (2010). Potential roles of anaerobic ammonia and methane oxidation in the nitrogen cycle of freshwater wetland ecosystems. Appl Microbiol Biotechnol 86: 1043–1055.
Zhu G, Peng Y, Li B, Guo J, Yang Q, Wang S . (2008). Biological nitrogen removal from wastewater. Rev Environ Contam Toxicol 192: 159–195.
Acknowledgements
We would like to thank Profs Junxin Liu, Jizheng He, Min Yang, Bongkeun Song and Weidong Wang for their kind help; Xien Long, Min Gao and Dong Li are gratefully acknowledged for their detailed and patient favor in molecular experiments. This research is financially supported by the National Natural Science Foundation of China (No.20877086), Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-EW-410-01), and the anammox research of Mike Jetten is supported by ERC Advanced Grant 232937. Dr Nils. Risgaard-Petersen acknowledges funding by Danish National Research Foundation and the German Max Plank Society.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, G., Wang, S., Wang, Y. et al. Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J 5, 1905–1912 (2011). https://doi.org/10.1038/ismej.2011.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2011.63
Keywords
This article is cited by
-
Redox controls on anaerobic ammonium oxidation coupled to reduction of natural organic matter in paddy ecosystems
Biology and Fertility of Soils (2023)
-
Quantitative Responses of Active Ammonia-Oxidizing Archaea and Bacteria to the Biological and Abiotic Factors Across Functional gene Distribution in Coastal Wetlands
Wetlands (2023)
-
Responses of anaerobic ammonia-oxidizing bacteria and methane-oxidizing archaea communities from different tillage modes in paddy fields
Journal of Soils and Sediments (2023)
-
Volatile fatty acids changed the microbial community during feammox in coastal saline-alkaline paddy soil
Environmental Science and Pollution Research (2023)
-
Artificial ponds as hotspots of nitrogen removal in agricultural watershed
Biogeochemistry (2022)