Abstract
Viruses were earlier found to be 10-fold more abundant than prokaryotes in deep granitic groundwater at the Äspö Hard Rock Laboratory (HRL). Using a most probable number (MPN) method, 8–30 000 cells of sulphate-reducing bacteria per ml were found in groundwater from seven boreholes at the Äspö HRL. The content of lytic phages infecting the indigenous bacterium Desulfovibrio aespoeensis in Äspö groundwater was analysed using the MPN technique for phages. In four of 10 boreholes, 0.2−80 phages per ml were found at depths of 342–450 m. Isolates of lytic phages were made from five cultures. Using transmission electron microscopy, these were characterized and found to be in the Podoviridae morphology group. The isolated phages were further analysed regarding host range and were found not to infect five other species of Desulfovibrio or 10 Desulfovibrio isolates with up to 99.9% 16S rRNA gene sequence identity to D. aespoeensis. To further analyse phage–host interactions, using a direct count method, growth of the phages and their host was followed in batch cultures, and the viral burst size was calculated to be ∼170 phages per lytic event, after a latent period of ∼70 h. When surviving cells from infected D. aespoeensis batch cultures were inoculated into new cultures and reinfected, immunity to the phages was found. The parasite–prey system found implies that viruses are important for microbial ecosystem diversity and activity, and for microbial numbers in deep subsurface groundwater.
Similar content being viewed by others
Introduction
Viruses are known to influence prokaryotic mortality and biogeochemical cycles, and to have great genetic diversity in marine ecosystems (Fuhrman, 1999; Suttle, 2005). They are the most abundant biological agents on earth, so microbial ecology in all environments is greatly affected by viruses. Deep subsurface water in granitic rock at the Äspö Hard Rock Laboratory (HRL) contained 104–106 cells ml–1 (Pedersen, 2001) and, in fact, the mass of subsurface prokaryotes likely exceeds the mass of plant and prokaryotic life in surface environments (Whitman et al., 1998). Kyle et al. (2008) have reported fluorescent microscopy counts of virus-like particles in the range of 105–107 ml−1 groundwater at the Äspö HRL from depths of 69–455 m and a morphologically diverse viral population when viewed under a transmission electron microscope (TEM). The number of viruses in Äspö groundwater exceeded the number of cells by one order of magnitude, a ratio frequently found in active surface environments (Kutter and Sulakvelidze, 2005; Suttle, 2005).
The lack of a large microbial biomass concentration in the intraterrestrial environments has commonly been taken as evidence that the microorganisms present are inactive or metabolizing extremely slowly (Kerr, 2002). The presence of viruses offers an alternate explanation, according to which viruses control active microbial populations in deep intraterrestrial environments. Viral lysis of bacterial cells releases nutrients, making an important contribution to microbial metabolism in benthic deep-sea ecosystems (Danovaro et al., 2008). However, the activity of such lytic viruses in deep granitic groundwater has yet to be confirmed by means of cultivation and isolation.
Deep groundwater in granitic rock is anaerobic and contained several metabolic groups of microorganisms, including sulphate-reducing bacteria (SRB) (Hallbeck and Pedersen, 2008). Viruses found in the natural environments were often species specific (Suttle, 2005) and could be isolated from environments where the host was found. Bacteriophages (phages) infecting three sulphate-reducing species of Desulfovibrio have been previously reported: Rapp and Wall (1987) found phages that could mediate transduction in Desulfovibrio desulfuricans; mitomycin C-induced lysogenic phages have been isolated from Desulfovibrio vulgaris by Handley et al. (1973); and Walker et al. (2006) and Kamimura and Araki (1989) isolated a lytic phage infecting Desulfovibrio salexigens.
The activity of phages in Äspö HRL groundwater was tested here using Desulfovibrio aespoeensis as a host. This bacterium was isolated from the Äspö HRL and described ∼10 years ago (Motamedi and Pedersen, 1998). It has been repeatedly enriched from Äspö HRL groundwater and identified by its 16S rRNA gene sequence (Pedersen et al., 1996; Fru and Athar, 2008). There was consequently strong evidence that D. aespoeensis is an indigenous species in the deep groundwater of the Äspö HRL. Here we report on the extent of phages infecting D. aespoeensis in the Äspö HRL, analysed with a most probable number (MPN) technique. Ten boreholes ranging in depth from 183 to 455 m were investigated. Phages were isolated from MPN cultures and described with respect to morphology and host range using six different species of Desulfovibrio, as well as ten different isolates of SRB from Äspö HRL groundwater. Growth of SRB and phage batch cultures was followed and the immunity of the cells to phage isolates was analysed.
Materials and methods
Sampling site: the Äspö HRL
The Äspö HRL was built as a research laboratory and to demonstrate the potential for the geological disposal of spent nuclear waste (Pedersen, 2001). Along the walls of the tunnel, groundwater-containing fractures are intersected by boreholes with packed-off borehole sections that could be accessed through valves and tubes. The age and origin of the water surrounding the tunnel have been modelled from geochemical data and were shown to correlate generally with salinity (Laaksoharju et al., 1999). The water was found to be heterogeneously distributed at different depths: water down to a depth of ∼250 m was dominated by meteoric freshwater, unlike water from depths of 250 to 600 m, which consisted of brackish–saline water with mixing proportions of current and ancient Baltic Sea water and meltwater from the last glaciation event ∼10 000 years ago (Supplementary information gives more details).
MPN of SRB and identification of SRB isolates
Sulphate-reducing bacteria were sampled from seven boreholes in the tunnel and enumerated by a MPN technique as described in Supplementary information. Four different isolates of SRB from Äspö groundwater were used to scan for the viable number of viruses and to analyse the host range of the isolated phages, as described in more detail below. Six additional Äspö groundwater SRB isolates were subsequently used to further test the phage host range. The ten Äspö groundwater isolates were identified by their 16S rRNA gene sequence as described in Supplementary information. Further, enterobacterial intergenomic repetitive consensus (ERIC) sequences from the ten isolate genomes were amplified by ERIC-PCR (Debruijn, 1992) as described in the Supplementary information.
Determination of total bacterial and viral numbers and viable biomass
The total number of cells and the number of virus-like particles were determined using a direct count method with SYBR Gold (Invitrogen, Eugene, OR, USA) according to Noble and Fuhrman (1998) and Chen et al. (2001). The concentration of viable biomass in cultures was estimated using an ATP assay (Lundin et al., 1986; Lundin, 2000). Details are given in Supplementary information. The s.d. of three ATP determinations of laboratory cultures typically ranged from 10 to 25% of the obtained mean value (Eydal and Pedersen, 2007).
Detection of lysed cultures
Three different methods for the detection of lysis were tested and compared on D. aespoeensis cultures immune and sensitive to the phages as described in detail in Supplementary information. The first method comprised visual inspection, the second method used the ATP assay to estimate the viable biomass in samples (Eydal and Pedersen, 2007) and the third method was to measure turbidity using spectrophotometry.
Sample collection of phages in the Äspö tunnel
Samples were collected from 10 boreholes along the Äspö HRL tunnel (Table 1) ranging in depth from 183 to 455 m, using in situ pressure and based on the descriptions by Pedersen (2001). Groundwater was drained from the borehole sections before sampling with a volume corresponding to one borehole volume. For boreholes KA2198A, KA2162B, SA1328A and KF0069A01, the groundwater was sampled from continuously flowing water. Samples for estimating viable numbers of virus (VNV) were collected using 20-ml BD Plastipak syringes (VWR) and filtered though 32-mm diameter, 0.2-μm pore size Filtropur S syringe filters (No./REF 83.1826.001; Sarstedt), before being transferred into N2-filled 27-ml anaerobic tubes using a needle. The filtration removed microbial cells from the water, but not viral particles smaller than 200 nm. The tubes containing the filtered groundwater were then moved to a subsurface laboratory at a depth of 450 m for addition to SRB batch cultures in mid-exponential growth and estimation of VNV.
Viable numbers of virus in Äspö HRL groundwater
All inoculations of borehole groundwater listed in Table 1 for the determination of VNV took place within 1 h of collection. Screening for phages infecting D. aespoeensis type strain Aspo-2 (DSM 10631) was conducted in October 2006 (Supplementary Table 1). In this screening, 5 ml of filtered groundwater, sampled as described for bacteriophage collection, was added using 10-ml BD Plastipak syringes (VWR) to triplicate 50-ml batch cultures of D. aespoeensis at mid-exponential growth. Cultures were grown for 2–3 weeks after sample inoculation, when visual inspection was used to detect infected cultures.
In addition, in November 2006 and March 2007, the VNV infecting D. aespoeensis in groundwater from boreholes, KA2162B, KA3110, KJ0052F03 and KA3510A, were estimated using a phage MPN technique in 10-ml portions of medium in 27-ml anaerobic tubes (Supplementary Table 1). Lysis and death of the host cultures was detected using visual inspection and the ATP assay. Groundwater filtered through 0.02-μm pore size Anodisc filters (Whatmann, Maidstone, UK) and added to cell cultures served as a control for SRB growth. This would also confirm that the agent responsible for culture lysis was between 20 and 200 nm in size.
The four SRB isolates from borehole KJ0052F01 (SRB2, SRB3, SRB5 and SRB22; Table 2) were considered potential hosts for phages from the Äspö HRL. To test this, groundwater from the ten boreholes listed in Table 1 were added to SRB cultures in September 2007. Six-replicate batch cultures of each of the four SRB isolates were grown in 9-ml portions in 27-ml anaerobic tubes and D. aespoeensis was used as the control. At mid-exponential growth, 1 ml of sample filtered though 0.2-μm pore size Filtropur S syringe filters (Sarstedt) was added to five cultures of each type. The sixth culture was used as a control to which sample filtered through 0.02-μm pore size Anodisc filters (Whatmann) was added. After adding the water samples, the cells were grown for 14 days. Cultures were analysed using visual inspection and spectrophotometric turbidity measurement to detect lysed cultures.
Phage isolates and phage morphology
Five phage isolates were obtained from infected D. aespoeensis cultures. Lysed cultures were chosen from cultures derived from the four boreholes, KA2162B, KA3110, KJ0052F03 and KA3510A, and diluted for the purification of phages; 1 ml of each lysed culture was filtered though a Filtropur S syringe filter with a 0.2-μm pore size (Sarstedt) and transferred to 9 ml of medium using 5-ml BD Plastipak syringes (VWR). Ten-fold serial dilutions were further made in 9 ml of medium using 1-ml BD Plastipak syringes (VWR), and 1 ml of each dilution was added to D. aespoeensis batch cultures during mid-exponential growth. Lysis of cultures was detected using visual inspection. The process of purification by dilution was repeated with the highest dilutions that displayed lysis, which were then retained and considered pure isolates. Phage morphology was examined to examine the morphology of the obtained phages and to confirm that the phage cultures retrieved had uniform morphologies as described in Supplementary information.
Host range experiments with phages on SRB
Six Desulfovibrio species that were widely distributed in the phylogenetic tree based on the 16S rRNA gene for Desulfovibrio (Bale et al., 1997) were exposed to phages to test the specificity of the five phage isolates. The six species included were retrieved from the German Collection of Microorganisms and Cell Cultures (DSMZ), and were D. aespoeensis subspecies Aspo-2 (DSM 10631), D. desulfuricans subspecies desulfuricans (DSM 642), D. vulgaris subspecies vulgaris (DSM 644), Desulfovibrio africanus (DSM 2603), D. salexigens (DSM 2638) and Desulfovibrio profundus (DSM 11384). These species were cultured in 9-ml portions in 27-ml anaerobic tubes. Each species was exposed to each phage isolate in triplicate by adding 1 ml of each culture of the five phage isolates filtered though 0.2-μm pore size Filtropur S syringe filters (Sarstedt). Triplicate controls containing only SRB batch cultures were also used. Cultures of D. aespoeensis infected with phages served as positive controls of infection. After addition of phages, the cells were grown for 11–15 days before analysis. The cell density of the cultures and the presence of lysis were measured using visual inspection and the ATP assay.
The host range of phages was further investigated using the four isolates from borehole KJ0052F01 (Table 2) and the six isolates from boreholes, KA3110A, KA3510A and KA2162B, (Table 2) using the same procedure. Lysed cultures were identified using visual inspection and the ATP assay or turbidity measurements using spectrophotometry 8 or 18–24 days after the phages had been added.
Growth, infection and reinfection of D. aespoeensis batch cultures
To follow SRB batch culture growth and infection by phages, D. aespoeensis was grown in six-replicate batch cultures of 50 ml of medium in 100-ml bottles. When the batch cultures had reached mid- to late-exponential growth after 94 h, 2 ml of phage isolate HEy2 was added through 0.2-μm pore size Filtropur S syringe filters (Sarstedt) into three of the cultures. Uninfected triplicate SRB cultures were grown for comparison and triplicate bottles containing medium served as the negative controls. Samples of 1.5 ml were taken eleven times, the first after 22 h and the last after 576 h, using 2-ml BD Plastipak syringes (VWR). Samples were preserved in 2-ml Eppendorf tubes using 0.02-μm filtered 37% acid-free formaldehyde (Scharlau Chemie, Sentmenat, Spain) to a final concentration of 2% and kept at 4 °C until the total number of cells and number of virus-like particles were determined.
Phage isolates were reintroduced to surviving cells from previously infected D. aespoeensis batch cultures to test for cells immune to the phages. The tested cells were taken from four of the six-replicate D. aespoeensis batch cultures used to follow SRB growth and infection by phage isolates as described in the previous paragraph. The triplicate cultures originally infected with phage isolate HEy2 and one of the uninfected cultures were transferred to and grown in seven batch cultures of 9 ml of medium in 27-ml anaerobic tubes. At mid-exponential growth, 1 ml of each of the five phage isolates HEy1–5 was filtered through 0.2-μm pore size Filtropur S syringe filters (Sarstedt) and added to separate tubes. The remaining two tubes served as controls. At 8 days after the phages had been added, visual inspection and turbidity measurements using spectrophotometry were used to detect lysed cultures.
Nucleotide sequence accession numbers
The 16S rRNA gene sequences of the SRB strains that were isolated and sequenced in this study have been submitted to the DDBJ/EMBL/GenBank databases under accession No. FJ037679, FJ037680, FJ037681, FJ037688, FJ627779, FJ627780, FJ627781 and FJ627782.
Results
MPN of SRB and identification of SRB isolates
The two boreholes, KA3542G01 and KJ0050F01, contained 3000 and 130 viable cells of SRB per ml as determined using the MPN method (Table 1). Samples from KJ0052F01 contained 30 000 cells ml–1. The numbers of SRB found in boreholes, KA2162B, KA3110A, KA3510A and KJ0052F03, were between 8 and 2200 cells ml–1.
The MPN cultures were subsequently used to isolate SRB. Four isolates denoting SRB2, SRB3, SRB5 and SRB22 were obtained from borehole KJ0052F01 (Table 2); they were all isolated from MPN cultures diluted from 103 to 106 relative to the original sample. The SRB isolates, SRB35 and SRB40, were from borehole KA3510A groundwater, and, SRB34 and SRB41, from KA2162B (Table 2); they originated from MPN cultures diluted from 103 to 105 relative to the sampled groundwater. The 16S rRNA gene sequences of the isolates grouped in two identical clusters that were similar but not identical to that of the D. aespoeensis type strain Aspo-2. A 99.1 and 99.9% base pair identity was found between the two cluster sequences and the type strain sequence (Table 2). However, the isolates displayed different ERIC-PCR gel band patterns when compared with each other (data not shown). One additional isolate was closely related to D. desulfuricans with 99.6% base pair identity.
Viable numbers of virus in Äspö HRL groundwater
Of the ten groundwater samples tested for VNV in October 2006 using D. aespoeensis as host, samples from the four boreholes, KA2162B, KA3110A, KA3510A and KJ0052F03, from depths of 342–450 m displayed phage infection on screening (that is, lysed cultures). MPN cultivations of VNV performed with groundwater from these boreholes showed 0.2−80 ml−1 infectious units of viable viruses infecting the host D. aespoeensis (Table 1). The samples positive for viral infection were collected at sites containing chloride of 3050–9070 mg l–1, sulphate of 313–659 mg l–1 and sulphide of 0.006–0.104 mg l–1 concentrations (Table 1).
Groundwater from the ten boreholes in Table 1 was sampled again in September 2007 and added to SRB2, SRB3, SRB5, SRB22, and to D. aespoeensis, now used as a control, to analyse for phage infection. For the only samples found to be positive for viral infection, these dates were those in which groundwater from boreholes, KA3510A and KA3110A, had been added to D. aespoeensis. These boreholes were earlier shown to contain the highest VNV infecting D. aespoeensis (Table 1).
Phage isolates and phage morphology
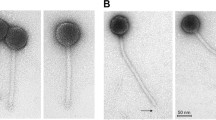
Five phage isolates, HEy1, HEy2, HEy3, HEy4 and HEy5, were isolated from infected D. aespoeensis cultures (Table 3). HEy3 was isolated from one 50-ml culture from borehole KA3510A and the other four isolates were isolated from the highest dilutions of the various MPN cultures: isolate HEy1 from borehole KA3510A, isolate HEy2 from KA3110, isolate HEy4 from KA2162B and isolate HEy5 from KJ0052F03. Figures 1a and b shows TEM images of the isolated phage. The morphology of the phage isolates was characterized as icosahedral heads with tails, and the phages belong to the Podoviridae morphology group and the C1 morphotype. The average head size (±s.d.) was 56.3 nm (±0.7, n=179). All isolates displayed a tail with an overall average length of 16.1 nm (±1.1, n=54) and width of 10.6 nm (±0.6, n=54) (Table 3). Phages attached to the cell surface of D. aespoeensis are shown in Figures 1c and d.
The morphologies of phages isolated from deep groundwater lytic to Desulfovibrio aespoeensis growing in a medium for sulphate-reducing bacteria are shown in transmission electron micrographs (a and b). Images (c and d) show phages (arrows) at the surface of a bacterium. Images were taken using × 70 000 magnification in (a−c) and × 45 000 magnification in (d). Images (a) and (b) are from phage isolate HEy5, (c) from isolate HEy4 and (d) from isolate HEy2. The scale bar is 100 nm in (a−c) and 500 nm in (d).
Host range experiments with phages on SRB
The host range test for infection with the six different DSMZ species showed the original host D. aespoeensis to be the only species receptive for lytic infection. Subsequently, the host range of the phage isolates was tested using the isolates, SRB2, SRB3, SRB5 and SRB22, from borehole KJ0052F01 (Table 2), all of which were resistant to phage infection. The host range of phage isolates was further tested using SRB32, SRB33, SRB34, SRB35, SRB40 and SRB41 isolated from boreholes, KA2162B, KA3110A and KA3510A, boreholes known from earlier experiments to contain phages lytic to D. aespoeensis. However, none of these cultures were infected. For isolate SRB32, the optical density values of the control cultures were low with greater variation, averaging (±s.d.) 0.062 (±0.076, n=3), making it difficult to judge whether this particular isolate had lysed. In addition, black precipitations had formed, so the cells might have lysed due to a lysogenic phage.
Growth, infection and reinfection of D. aespoeensis batch cultures
Batch cultures of D. aespoeensis grew exponentially for ∼120 h, before they reached the stationary growth phase at a concentration of 5–6 × 108 cells ml–1 (Figure 2). The cultures to which phage isolate HEy2 had been added after 94 h contained 5.3 × 107 cells ml–1 at the time of phage addition, and the number of cells per ml–1 decreased to 2.1 × 106 cells ml–1 after 263 h. Bacterial morphology indicated that the cells were enlarged after 119 h of growth and that many cells were destroyed after 143 h with viral particles found in close proximity. After 360 h, the cells had started to increase in number again and the culture had the same concentration of cells, as did the uninfected control cultures after 576 h. Few enlarged or lysed cells with viral particles in close proximity were observed in the 360- and 576-h samples. The number of phages in the infected batch cultures was 1.0 × 108 phages ml–1 at addition; this number increased to ∼2 × 1010 phages ml–1 after 167 h. Using the average numbers of virus-like particles before and after lysis, the viral burst size was calculated to be 170. The time taken by the phages to lyse most of the SRB cells, the latent period, was ∼70 h. The surviving cells from lysed D. aespoeensis cultures again exposed to phages were not lysed, whereas cells exposed to the phages for the first time were infected; this was observed for all HEy1–5 phage isolates.
Sulphate-reducing bacteria and phage numbers followed over time by counting particles filtered onto a 0.02-μm pore size filter and stained with SYBR Gold. Samples were taken from 50-ml uninfected (▴) and infected (▪) batch cultures of D. aespoeensis. Phage isolate HEy2 (□) was added after 94 h and the error bars indicate s.d. (n=3).
Discussion
Viable numbers of SRB and phages
The water around the Äspö HRL tunnel at a depth below 250 m is ancient anaerobic glacial water and seawater that has been subsurface for ∼10 000 years (Laaksoharju et al., 1999). The sampled groundwater contained sulphate of 313–659 mg l–1and sulphide of 0.006–2.01 mg l–1 concentrations (Table 1), which suggested that microbial sulphate reduction was ongoing. Hence, SRB could be cultivated from all tested samples. Although SRB could be cultivated in substantial numbers from the Äspö HRL groundwater, many microorganisms from environmental samples are difficult to cultivate and isolate. As viruses are normally species specific and need an isolated host in order to be cultivated, enumeration of infectious viruses in the groundwater is even more challenging. To the best of our knowledge, this is the first time viable viruses have been isolated from deep granitic groundwater, as well as the first time the numbers of viruses infectious to a Desulfovibrio species in environmental samples have been successfully estimated.
Applying a phage MPN method with D. aespoeensis as a host, groundwater from four of ten tested boreholes at depths ranging from 342 to 450 m was shown to contain active phages (Table 1). At these locations, or in the vicinity, the lytic release of phages was extensive enough to be detected using the MPN method. Reliable methods are needed to cultivate and detect viral infection of bacterial cultures. As the SRB used in this study grew well in liquid media, and plaque count methods are difficult to perform under anaerobic conditions for SRB, the MPN method in liquid media was used to estimate VNV. Such methods have been used extensively to trace viruses and monitor water quality, and the total cultivable virus assay MPN method is recommended by the US Environmental Protection Agency (Anonymous, 1995). The MPN method then allowed the number of infectious units to be compared between different sites at the Äpö HRL.
Phage morphology
From phage MPN cultures using D. aespoeensis as a host, phages were isolated and TEM was used to confirm the presence of pure phage cultures and to describe their morphology. The phage isolates were all in the Podoviridae morphology group with an average head diameter of 56 nm (Figure 1 and Table 3). Podoviruses are known to have subterminal fibres, but these structures were not observed, possibly because of the interference from the sulphide and salts in the medium. Podoviruses were also described by Kyle et al. (2008) using TEM on groundwater samples from borehole KA3110A in the Äspö HRL, the borehole here shown to have the highest MPN of phages. The morphologies of phages infecting three Desulfovibrio species have been previously reported. All of these earlier described phages were tailed, but differed from each other and from the phages isolated from the Äspö HRL. Rapp and Wall (1987) found phages that were able to mediate transduction in D. desulfuricans. These phages, like those reported here, belonged to the Podoviridae morphology group, having a head ∼43 nm in diameter and a tail 7.1 nm long and 5.7 nm wide. Other types of phages that have been reported to infect Desulfovibrio are Myoviridae (Handley et al., 1973), icosahedral phages (Walker et al. 2006) and Siphoviridae (Kamimura and Araki 1989). Although only a few different phages have been isolated so far with Desulfovibrio species as a host, more phages are likely to be described.
Host range and selection of immune cells
To further describe the phages isolated from Äspö HRL groundwater, their host range was tested. Of the six Desulfovibrio type strains, only D. aespoeensis was infected. Subsequently, the next step was to add the phage isolates to cultures of SRB isolates from earlier experiments at the Äspö HRL. The D. aespoeensis isolates, SRB2, SRB3, SRB5 and SRB22, from borehole KJ0052F01 were shown to be 99.1% similar to D. aespoeensis in their 16S rRNA gene sequence, though the ERIC-PCR patterns differed between the isolates. All these isolates were found to be immune to phage isolates HEy1–5, and the phages were likely to be specific to D. aespoeensis aspo-2 type strain only.
As the phages were found in groundwater originating from four different boreholes at the Äspö HRL (KJ0052F03, KA2162B, KA3110A and KA3510A), their host was likely to live at the same locations. From SRB cultivations from these boreholes, five isolates of SRB similar in 16S rRNA gene sequence to D. aespoeensis and one isolate similar to D. desulfuricans were retrieved (Table 2). Surprisingly, none of these isolates was infected with the phage isolates, even though the isolates had 99.9% 16S rRNA gene sequence identity to D. aespoeensis. Podoviruses have often been found abundantly in oceans and to have a narrow host range (Suttle, 2005), which is in line with the host range found in the present study. On the other hand, as only isolates from high dilutions of groundwater were tested here, it may be merely by chance that no isolates sensitive to the phages were found. Alternately, these SRB isolates might be dominant in the boreholes because they were in fact resistant to the phages in the water (Waterbury and Valois, 1993). The host range of phages can also be influenced by how they were isolated, as was shown by Jensen et al. (1998), working mainly with samples from sewage sources. They isolated a higher frequency of phages with a broader host range when two bacterial strains were used as hosts to isolate phages. It is unknown to what extent of cultivation of SRB from borehole groundwater might have selected for bacteria immune to the phages common in these environments—cells might easily become immune to phages as shown here (Figure 2).
The selection of immune cells was further investigated in the laboratory. When bacterial and viral numbers were followed in batch cultures, most of the cells exposed to the phage isolate HEy2 were lysed, with a latent period of ∼70 h and a viral burst size of 170 (Figure 2). The viral burst size has been shown to increase with the cell size of the host in marine samples (Weinbauer and Peduzzi, 1994). This would mean that factors affecting the cell size, such as the nutrient supply, might determine the number of released viral particles. Deep terrestrial ecosystems are oligotrophic, where the number of viruses produced by a viral infection is likely to be smaller than that observed in laboratory batch cultures. After the first phage lysis observed in the D. aespoeensis Aspo-2 laboratory batch cultures, some of the cells started to grow again in the three parallel cultures (Figure 2). The cells that were able to grow despite the presence of phages were inoculated into fresh medium. By adding the five phage isolates, HEy1–5, to the cultures of cells surviving the HEy2 phage isolate attack, D. aespoeensis cultures were found to be immune to all the five phage isolates. This indicates that all the phage isolates originating from four different locations at the Äspö HRL infect D. aespoeensis using a similar mechanism. It may be that some of the phages entered a lysogenic cycle and were integrated into the genome of their host, which by this mechanism became immune to the same type of phage.
On the basis of what has been learnt so far about the five phages isolated and described here, they seemed very similar to each other, though the degree of identity between the isolates needs to be tested by comparison of their DNA sequences to confirm that they are in fact the same phage. By selecting for cells immune to viruses, microorganisms and viruses from deep groundwater environments can evolve over time through an ‘arms race’ between viruses and bacteria. A similar replacement of cells sensitive to the phages with resistant clones has been observed in marine phage–host systems grown in artificial seawater batch cultures (Middelboe et al., 2001).
The SRB isolated from Äspö HRL groundwater in this study displayed variation in the 16S rRNA gene sequence and ERIC-PCR pattern. The bacterial communities have likely changed and diverged over time and phage activity might have contributed to this development. This could have happened as described by the ‘killing the winner’ theory (Thingstad and Lignell, 1997), according to which lytic phages kill the fast-growing and dominant cells, enabling the coexistence of less competitive populations and thereby sustaining bacterial diversity. The phages from the Äspö HRL might also affect the microbial evolution by mediating gene transfer between cells, as described by Weinbauer and Rassoulzadegan (2004). Rapp and Wall (1987) found phages that were able to mediate transduction in D. desulfuricans, and the parasite–prey system of phages and bacteria described here supports the idea that transduction could occur in the subsurface environment.
The phages isolated here had a narrow host range, and a morphologically diverse viral population (including polyhedral, tailed, filamentous and pleomorphic viruses) has previously been observed in Äspö HRL groundwater (Kyle et al., 2008). Taken together, this suggests that a great many types of viruses may be involved in controlling microbial populations. All types of viruses present must be considered when modelling the evolution of deep biosphere ecosystems and when studying the activity of their microbial populations.
Intraterrestrial environments, represented here by the Äspö HRL, have been argued to be inhabited by inactive or extremely slowly metabolizing microorganisms because of the lack of a large microbial biomass (Kerr, 2002). Like what was found in deep-sea sediments (Danovaro et al, 2008), the finding of active lytic viruses offers an alternate explanation where the microorganisms are in a state of growth. This supports the earlier data obtained from significant energy source assimilation and activity (Pedersen and Ekendahl, 1992) and amounts of ATP in groundwater (Eydal and Pedersen, 2007).
As the specific host must be replicating and present in numbers that are able to sustain the viruses, the idea of the viruses as isolated from dynamic microbial communities is further supported by the narrow viral host range. As well, the isolated viruses are lytic, which is the life strategy thought to be more common when the host density is high (Chibani-Chennoufi et al., 2004). This stands in contrast to lysogeny, which has been shown to be more prevalent in isolates from oligotrophic marine environments induced with mitomycin C and UV radiation (Jiang and Paul, 1998).
References
Anonymous (1995) Agency USEP (ed) Virus monitoring protocol for the information collection requirements rule. Government Printing Office: Cincinnati, OH.
Bale SJ, Goodman K, Rochelle PA, Marchesi JR, Fry JC, Weightman AJ et al. (1997). Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol 47: 515–521.
Chen F, Lu JR, Binder BJ, Liu YC, Hodson RE . (2001). Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR gold. Appl Environ Microbiol 67: 539–545.
Chibani-Chennoufi S, Bruttin A, Dillmann ML, Brussow H . (2004). Phage–host interaction: an ecological perspective. J Bacteriol 186: 3677–3686.
Danovaro R, Dell’Anno A, Corinaldesi C, Magagnini M, Noble R, Tamburini C et al. (2008). Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 454: 1084–1087.
Debruijn FJ . (1992). Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain-reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol 58: 2180–2187.
Eydal HSC, Pedersen K . (2007). Use of an ATP assay to determine viable microbial biomass in Fennoscandian Shield groundwater from depths of 3–1000 m. J Microbiol Methods 70: 363–373.
Fru EC, Athar R . (2008). In situ bacterial colonization of compacted bentonite under deep geological high-level radioactive waste repository conditions. Appl Microbiol Biot 79: 499–510.
Fuhrman JA . (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399: 541–548.
Hallbeck L, Pedersen K . (2008). Characterization of microbial processes in deep aquifers of the Fennoscandian Shield. Appl Geochem 23: 1796–1819.
Handley J, Adams V, Akagi JM . (1973). Morphology of bacteriophage-like particles from Desulfovibrio vulgaris. J Bacteriol 115: 1205–1207.
Jensen EC, Schrader HS, Rieland B, Thompson TL, Lee KW, Nickerson KW et al. (1998). Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl Environ Microbiol 64: 575–580.
Jiang SC, Paul JH . (1998). Significance of lysogeny in the marine environment: studies with isolates and a model of lysogenic phage production. Microb Ecol 35: 235–243.
Kamimura K, Araki M . (1989). Isolation and characterization of a bacteriophage lytic for Desulfovibrio salexigens, a salt-requiring, sulfate-reducing bacterium. Appl Environ Microbiol 55: 645–648.
Kerr RA . (2002). Deep life in the slow, slow lane. Science 296: 1056–1058.
Kutter E, Sulakvelidze A . (2005). Bacteriophages: Biology and Applications. CRC Press: Boca Raton, FL.
Kyle JE, Eydal HSC, Ferris FG, Pedersen K . (2008). Viruses in granitic groundwater from 69 to 450 m depth of the Äspö Hard Rock Laboratory, Sweden. ISME J 2: 571–574.
Laaksoharju M, Tullborg EL, Wikberg P, Wallin B, Smellie J . (1999). Hydrogeochemical conditions and evolution at the Äspö HRL, Sweden. Appl Geochem 14: 835–859.
Lundin A . (2000). Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Meth Enzymol 305: 346–370.
Lundin A, Hasenson M, Persson J, Pousette A . (1986). Estimation of biomass in growing cell-lines by adenosine triphosphate assay. Meth Enzymol 133: 27–42.
Middelboe M, Hagstrom A, Blackburn N, Sinn B, Fischer U, Borch NH et al. (2001). Effects of bacteriophages on the population dynamics of four strains of pelagic marine bacteria. Microb Ecol 42: 395–406.
Motamedi M, Pedersen K . (1998). Desulfovibrio aespoeensis sp. nov., a mesophilic sulfate-reducing bacterium from deep groundwater at Äspö hard rock laboratory, Sweden. Int J Syst Bacteriol 48: 311–315.
Noble RT, Fuhrman JA . (1998). Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol 14: 113–118.
Pedersen K . (2001). Diversity and activity of microorganisms in deep igneous rock aquifers of the Fennoscandian Shield. In: Fredrickson JK, Fletcher M (eds). Subsurface Microgeobiology and Biogeochemistry. Wiley-Liss: New York, pp 97–139.
Pedersen K, Arlinger J, Ekendahl S, Hallbeck L . (1996). 16S rRNA gene diversity of attached and unattached bacteria in boreholes along the access tunnel to the Äspö Hard Rock Laboratory, Sweden. FEMS Microbiol Ecol 19: 249–262.
Pedersen K, Ekendahl S . (1992). Assimilation of CO2 and introduced organic compounds by bacterial communities in ground water from Southeastern Sweden deep crystalline bedrock. Microb Ecol 23: 1–14.
Rapp BJ, Wall JD . (1987). Genetic transfer in Desulfovibrio Desulfuricans. Proc Natl Acad Sci USA 84: 9128–9130.
Suttle CA . (2005). Viruses in the sea. Nature 437: 356–361.
Thingstad TF, Lignell R . (1997). Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol 13: 19–27.
Walker CB, Stolyar SS, Pinel N, Yen HCB, He ZL, Zhou JZ et al. (2006). Recovery of temperate Desulfovibrio vulgaris bacteriophage using a novel host strain. Environ Microbiol 8: 1950–1959.
Waterbury JB, Valois FW . (1993). Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol 59: 3393–3399.
Weinbauer MG, Peduzzi P . (1994). Frequency, size and distribution of bacteriophages in different marine bacterial morphotypes. Mar Ecol Prog Ser 108: 11–20.
Weinbauer MG, Rassoulzadegan F . (2004). Are viruses driving microbial diversification and diversity? Environ Microbiol 6: 1–11.
Whitman WB, Coleman DC, Wiebe WJ . (1998). Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583.
Acknowledgements
We wish to thank Johanna Arlinger, Lisa Karlsson and Sara Eriksson at Microbial Analytics Sweden AB for assistance with laboratory cultivations and with sampling at the Äspö HRL, and Jennifer Kyle, Grant Ferris, Mikal Heldal and Gunnar Bratbak for assistance with TEM. We are also grateful for funding from the Swedish Nuclear Fuel and Waste Management Co. and the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Eydal, H., Jägevall, S., Hermansson, M. et al. Bacteriophage lytic to Desulfovibrio aespoeensis isolated from deep groundwater. ISME J 3, 1139–1147 (2009). https://doi.org/10.1038/ismej.2009.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2009.66
Keywords
This article is cited by
-
The Fennoscandian Shield deep terrestrial virosphere suggests slow motion ‘boom and burst’ cycles
Communications Biology (2021)
-
Energy efficiency and biological interactions define the core microbiome of deep oligotrophic groundwater
Nature Communications (2021)
-
Corrosion Inhibition of X80 Steel in Simulated Marine Environment with Marinobacter aquaeolei
Acta Metallurgica Sinica (English Letters) (2019)
-
The biomass and biodiversity of the continental subsurface
Nature Geoscience (2018)
-
The deep continental subsurface: the dark biosphere
International Microbiology (2018)