Abstract
Ammonium concentration and nitrogen source regulate promoter activity and use for the transcription of chiA, the major chitinase gene of Pseudoalteromonas sp. S91 and S91CX, an S91 transposon lacZ fusion mutant. The activity of chiA was quantified by β-galactosidase assay of S91CX cultures containing different ammonium concentrations (NH4+; 0, 9.5 or 191 mM) and with different nitrogen sources (N-acetylglucosamine (GlcNAc) or glutamate (glt)). S91 chiA expression was found to depend on both the NH4+ concentration and source of nitrogen in marine minimal medium (MMM). Pseudoalteromonas sp. S91 and S91CX can use either GlcNAc or glt as a sole source of carbon in MMM containing a standard concentration of 9.5 mM NH4+. Adding excess NH4+, 20 times the standard concentration, to MMM significantly reduced chiA activity below that found in the presence of either GlcNAc or glt. When no NH4+ was added to MMM, S91CX was also able to use either GlcNAc or glt as a source of nitrogen; under these conditions chiA activity was significantly increased. Under all conditions tested, GlcNAc induced chiA activity significantly more than glt. Regulation of bacterial chitinases by nitrogen has not been previously reported. Transcriptional start point analysis of S91 chiA, using 5′RACE (ligation-anchored PCR), showed that during growth in MMM supplemented with (1) maltose (solely a carbon source for S91), chiA transcription occurred from only one putative σ70-dependent promoter; (2) the chitin monomer GlcNAc, transcription initiated from two putative σ54-dependent promoters and (3) glt, transcription initiated from all three putative promoters.
Similar content being viewed by others
Introduction
The marine ecosystem relies on bacteria to maintain balanced nitrogen pools, as directly utilizable dissolved organic nitrogen is only available at low concentrations (Cottrell and Kirchman, 2000). The majority of the oxidation–reduction reactions, which transform nitrogen to different forms within the nitrogen cycle, are mediated by bacteria, which utilize the nitrogen as an energy source (Zehr and Ward, 2002). Chitin, an insoluble polysaccharide of N-acetylglucosamine (GlcNAc) monomers, is one of the most abundant carbon and nitrogen sources for marine bacteria (Gooday, 1990; Keyhani and Roseman, 1999). Chitinolytic bacteria secrete extracellular chitinases that degrade chitin into small oligosaccharides and GlcNAc subunits, which are imported and directly utilized by cells (Wetzel, 1991), a process which is essential for oceanic carbon, nitrogen and energy recycling (Zehr and Ward, 2002). The particulars of the role of bacteria in the marine nitrogen cycle, however, such as the regulation of genes involved in the assimilation and utilization of large organic nitrogenous compounds such as chitin, are not fully understood (Zehr and Ward, 2002). New research investigating the genetics and regulation of microbial enzymes involved in nitrogen cycle transformations is essential for an increased understanding of the nitrogen cycle, the processes, their relatedness and interaction (Zehr and Ward, 2002); understanding of the carbon cycle, although not complete, is more advanced.
Pseudoalteromonas sp. S91 is a chitinolytic marine bacterium (Techkarnjanaruk et al., 1997) in which the chitinase gene cluster, chiABC, is transcribed as an operon as well as separately from individual promoters possessed by each gene (Delpin and Goodman, 2009). In the transposon mutant strain S91CX, which has a lacZ reporter gene inserted under the control of the chiA promoter region, chiA promoter activity is regulated by growth conditions (Techkarnjanaruk et al., 1997). A basal level of chiA activity was found for cells grown in a marine minimal medium (MMM) supplemented with glutamate (glt) as the carbon source; chiA was induced by the addition of either chitin or GlcNAc (Techkarnjanaruk et al., 1997).
S91 chiA potentially has one σ70- and two σ54-dependent promoters, all of which are active during growth in MMM supplemented with glt with or without 0.1% (5 mM) GlcNAc (Delpin and Goodman, 2009). Many σ54-dependent promoters have been found to be part of the nitrogen regulatory system (Zimmer et al., 2000). In the literature, although chitin is often referred to as a source of carbon and nitrogen, studies have focused on chitin as a carbon source. To date there are no studies of chitin being used as the sole nitrogen source for bacteria, and no reports of bacterial chitinase genes being regulated by nitrogen. This study investigated the effects of nitrogen on S91 chiA gene regulation and found that chiA expression was dependent on nitrogen concentration (ammonium) as well as source (GlcNAc or glt).
Materials and methods
Bacterial strains and growth conditions
Pseudoalteromonas sp. S91 (Albertson et al., 1996) and S91CX (Techkarnjanaruk et al., 1997) were grown with shaking at 30 °C in tryptone soya broth (TSB; Oxoid, Bastingstoke, Hampshire, UK) supplemented with 20 g l−1 NaCl, 1 mM MgCl2 and 300 μM CaCl2, or MMM (Östling et al., 1991), which contains 9.5 mM NH4Cl as standard. MMM with no added NH4Cl (MMM NN) or with 20 times (191 mM) the standard concentration of NH4Cl (MMM 20 N) was also used. MMM plus 0.2% (w/v) maltose (MMM maltose) was used as the basal minimal growth medium; NH4+ and maltose in MMM provided nitrogen and carbon, respectively. Glutamate (40 mM) and GlcNAc (5 mM) provided both nitrogen and carbon.
Comparative growth analyses
S91 wild-type (wt) and S91CX were inoculated separately into 100 ml Erlenmeyer flasks containing 10 ml of MMM maltose and grown for 24 h with shaking. Each overnight culture was diluted 1:10 into 100 ml side-armed flasks in duplicate, each containing 20 ml of either MMM NN GlcNAc, MMM GlcNAc, MMM NN glt, MMM glt, MMM NN maltose or MMM maltose. Cultures were grown with shaking and their optical density (595 nm; Milton Roy Spectronic 20+) was recorded hourly over 6 h, using the corresponding sterile medium as the blank. There were no growth differences between S91 wt and S91CX (data not shown). Both strains were able to use either GlcNAc or glt as a nitrogen and a carbon source as they were able to grow in MMM NN with either of these. As expected, neither S91 wt nor S91CX was able to grow in MMM NN maltose, due to the absence of a nitrogen source.
Assay of chitinase gene expression
Measurement of β-galactosidase-specific activity (sp. act.) levels enabled quantification of the chiA promoter activity in S91CX following growth under different nutrient regimes (Techkarnjanaruk et al., 1997). An overnight S91CX culture was diluted 1:100 from the corresponding medium into either MMM NN GlcNAc, MMM GlcNAc, MMM 20 N GlcNAc, MMM NN glt, MMM glt, MMM 20 N glt or MMM maltose. Cultures were grown for 24 h with shaking. β-Galactosidase activity assays and specific activity calculations were as detailed in Stretton et al. (1996); sp. act. was defined as μmol o-nitrophenol released per min (mg protein)−1 (Miller, 1972).
Transcriptional start point analysis
S91 wt was inoculated into a 100 ml Erlenmeyer flask containing 10 ml of MMM maltose and grown for 24 h with shaking. The overnight culture was diluted 1:100 into either 10 ml MMM GlcNAc, MMM NN GlcNAc, MMM maltose or MMM glt and grown with shaking. Cultures of OD595 0.7–0.8 were treated with RNA protect (Qiagen, Venlo, Netherlands) and RNA was isolated using an RNeasy minikit (Qiagen) as described in detail by Delpin and Goodman (2009). Reverse transcription (RT)-PCR of S91 chiA was carried out as described in Delpin and Goodman (2009) using Thermoscript reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and the chiA gene-specific primer chiA-R895. RNA was removed from transcribed cDNA by alkaline hydrolysis followed by glycogen precipitation, to recover the cDNA (Delpin and Goodman, 2009). Successful cDNA synthesis was checked by PCR; the primer pair used was chiA-F776 and chiA-R895 (Delpin and Goodman, 2009).
Ligation-anchored (LA)-PCR was used to locate the base at which chiA transcription initiated under the different growth conditions tested. LA-PCRs and the analyses of products were carried out as described in detail by Delpin and Goodman (2009). Briefly, a chiA gene-specific primer, chiA-R2 was used with the DT88 anchor oligonucleotide-specific primer, DT89, for LA-PCR1 (Tillett et al., 2000; Delpin and Goodman, 2009). For LA-PCR2, the chiA gene-specific primer chiA-R3, located within the LA-PCR1 product, was used with DT89 (Tillett et al., 2000; Delpin and Goodman, 2009). A negative control containing dH2O in place of DNA template was always included. LA-PCR setup and thermocycling conditions used are described in Delpin and Goodman (2009). LA-PCR2 products were excised following electrophoresis on 3% (w/v) agarose gels in Tris acetate EDTA buffer. Excised fragments were Wizard cleaned (Promega, Madison, WI, USA) and electrophoresed to check for purity and to quantify each fragment for sequencing (Flinders Medical Centre Sequencing Facility, Flinders University, Adelaide, Australia) using the ABI BigDye Terminator method (Applied Biosystems, Foster City, CA, USA). Local pair alignment (Huang and Miller, 1991) and Biomanager by ANGIS (http://www.angis.org.au) were used for nucleic acid sequence alignments to chiA and DT88 to determine at which base transcription initiated (Delpin and Goodman, 2009). Sequences of all PCR primers are given in Delpin and Goodman (2009).
Statistical analyses
β-Galactosidase assays were carried out in duplicate and each experiment was repeated at least four times. Differences between two means were compared by two-sample two-tailed t-tests (Zar, 1996).
Results and discussion
S91 chiA promoter activity
Both concentration (ammonium) and source (GlcNAc or glt) of nitrogen regulated S91CX chiA promoter activity. chiA activity was induced when MMM contained no ammonium (MMM NN) and was repressed when ammonium was added in excess (191 mM, MMM 20 N) in the presence of either GlcNAc or glt (Figure 1). The highest level of chiA promoter activity occurred following S91CX growth in MMM NN GlcNAc. This was significantly higher than that in MMM GlcNAc (P<0.05) and any other growth condition (Figure 1). When S91CX was grown in MMM 20 N GlcNAc, chiA activity was significantly lower compared to the level observed in standard MMM GlcNAc (P<0.05; Figure 1). This same trend was found for S91CX grown in glt (Figure 1); highest activity was found in MMM NN glt, growth in MMM glt produced significantly lower activity (P<0.05) and the lowest activity was in MMM 20 N glt.
In minimal medium, ammonia is the preferred nitrogen source for Escherichia coli as it is the end product of nitrogen metabolism (Reitzer and Schneider, 2001). The presence of ammonia represses the synthesis of several E. coli nitrogen metabolic proteins (Reitzer and Schneider, 2001). Similarly in S91CX, the presence of increasing concentrations of ammonium in MMM increasingly repressed chiA promoter activity (Figure 1). In MMM glt under conditions of nitrogen excess (191 mM), chiA activity (Figure 1) was as low as that reported previously for S91CX grown in rich medium (TSB; Techkarnjanaruk et al., 1997). When S91CX was grown in TSB, chiA levels were fivefold lower than when S91CX was grown in MMM glt, and chiA was said to be repressed (Techkarnjanaruk et al., 1997). It is possible that this repression reported by Techkarnjanaruk et al. (1997) was caused by a high concentration of nitrogen in the TSB medium.
In the nitrogen regulatory response, low environmental nitrogen concentration results in increased NtrC phosphorylation, leading to an increase in σ54-dependent promoter gene transcription (Magasanik, 1996; Atkinson et al., 2002). For example, microarray analysis has shown that expression of the E. coli astCADBE operon is increased 7- to 11-fold under nitrogen limitation (Zimmer et al., 2000). The astCADBE operon possesses a σ54-dependent promoter and encodes proteins of the arginine succinyltransferase pathway that catabolize arginine (Reitzer and Schneider, 2001). Such a trend was observed for S91CX chiA activity irrespective of the nutrient added; the lower the ammonium concentration, the higher the chiA promoter activity (Figure 1). S91 chiA possesses two putative σ54-dependent promoters from which transcription has been observed to initiate (Delpin and Goodman, 2009). It is possible therefore that NtrC, or an NtrC-like protein, may be the activator of the potential chiA σ54-dependent promoters. This needs to be further investigated.
Davalos et al. (2004) used whole-genome arrays to characterize the response of Sinorhizobium meliloti Ntr-responsive genes under various nutrient regimes. Of note, growth of S. meliloti in glt as a source of nitrogen, in place of ammonium, induced nitrogen catabolic genes (Davalos et al., 2004). Similarly, when S91CX was grown in glt in the presence of decreasing concentrations of ammonium, chiA was increasingly induced (Figure 1). With no added ammonium in the medium (MMM NN glt), glt was able to serve as the source of nitrogen for S91. This may explain why chiA was expressed significantly higher in cells grown in standard MMM with glt, a source of nitrogen, than with maltose, which does not contain nitrogen. That is, in MMM glt chiA appeared to be activated as part of the S91 Ntr response that recognized glt as a source of nitrogen. In MMM maltose, the presence of ammonium as the only source of nitrogen did not induce chiA, as the presence of ammonia represses the expression of nitrogen metabolic genes, as seen in E. coli (Reitzer and Schneider, 2001).
GlcNAc, the monomer of chitin, induced S91 chiA more so than glt. For each respective NH4+ concentration, chiA activity was significantly higher for S91CX grown in GlcNAc compared to glt (P<0.05; Figure 1). As discussed, the absence of ammonium in the presence of glt induces nitrogen catabolic genes in S. meliloti (Davalos et al., 2004). As the expression of chiA in MMM NN GlcNAc was significantly greater than that in MMM NN glt, it is possible that the strong induction of chiA by GlcNAc may be indicative of a further layer of regulation on chitinase genes, in addition to regulation as part of the nitrogen assimilation system. We are currently investigating whether either of the chiA σ54-dependent promoters is active in S91 cells grown in MMM GlcNAc with ammonium excess.
Techkarnjanaruk et al. (1997) considered chiA expression to be basal following growth in MMM glt. This study showed that chiA activity in S91CX cells grown in MMM maltose should be considered as the basal level of activity. The level of chiA activity for S91CX grown in MMM maltose was significantly lower than in MMM GlcNAc (P<0.05) or MMM glt (P<0.05) (Figure 1). With β-galactosidase sp. activity of 342 units, chiA activity in MMM maltose is about fivefold lower than that of cells grown in MMM glt (β-galactosidase sp. activity 1657 units; Figure 1; Techkarnjanaruk et al., 1997).
S91 chiA promoter transcription
N-acetylglucosamine
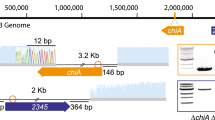
When S91 was grown in MMM GlcNAc or MMM NN GlcNAc, two chiA transcripts were detected in both media. LA-PCR2 generated two fragments of approximately 200 and 70 bp in length (Figures 2a and b; Table 1) and when sequenced, located a TSP to base 185 and base 300, respectively, of S91 chiA (GenBank accession no. AF007894); these were the same as TSPs A2 and A3, respectively, found previously (Delpin and Goodman, 2009). Under these growth conditions LA-PCR did not detect transcription from TSP A1, because if TSP A1 were active, a 310 bp LA-PCR2 fragment would have been expected (Delpin and Goodman, 2009). PCR was used to show that cDNA transcript upstream of TSP A2 was present; using forward primer chiA-F472 (18 bp downstream of TSP A1) and reverse primer chiA-R2, a PCR product of the expected length of about 300 bp was amplified in separate reactions using MMM NN GlcNAc and MMM GlcNAc chiA cDNA as template (data not shown). No PCR product was obtained using forward primer T8G17Eco-F (60 bp upstream of TSP A1) and chiA-R2. These data showed that transcription (at a level too low to be detected by the LA-PCR method) of chiA had occurred in the vicinity of TSP A1 under these growth conditions.
(a) TAE agarose gel (3%) of ligation-anchored (LA)-PCR2 reaction products (5 μl) of S91 chiA amplified from cDNA produced from RNA isolated from cells grown in marine minimal medium (MMM) NN GlcNAc. Lane 1, MMM NN GlcNAc; lane 2, reverse transcription (RT)-PCR-negative control of MMM NN GlcNAc; lane 3, 5 μl of a 50 ng μl−1 100 bp DNA ladder, 100 bp increments from 1000 to 100 bp. (b) TAE agarose gel (3%) of LA-PCR2 reaction products (5 μl) of S91 chiA amplified from cDNA produced from RNA isolated from cells grown in MMM GlcNAc. Lane 1, MMM GlcNAc; lane 2, RT-PCR-negative control of MMM GlcNAc; lane 3, 5 μl of a 50 ng μl−1 100 bp DNA ladder.
Following S91 growth in either MMM GlcNAc or MMM NN GlcNAc the presence or absence of NH4+ did not change TSP use. Under these growth conditions, the majority of S91 chiA transcription initiated from TSPs A2 and A3, each of which possess a putative σ54-dependent promoter (Delpin and Goodman, 2009). The majority of genes that possess a σ54-dependent promoter are involved in nitrogen metabolism (Reitzer and Schneider, 2001). Reitzer and Schneider (2001) concluded that the E. coli glnK gene possessed a σ54-dependent promoter on the basis that (1) nitrogen limitation increased glnK transcript quantity at least 10-fold, (2) there was a promoter with high homology to that of a σ54-dependent promoter and (3) there was an NtrC-binding site. An NtrC-binding site can be positioned up to 1 kb up- or downstream of a σ54-dependent promoter (Buck et al., 1986). No such binding site has yet been identified for S91 chiA; sequencing upstream of chiA is underway. It has been observed, however, that in the presence of high levels of NtrC-P, no DNA binding is required for transcriptional activation of the E. coli glnA σ54-dependent promoter (Reitzer and Magasanik, 1986; Schneider et al., 1991).
Maltose and glutamate
Following S91 growth in MMM maltose, only one TSP was used for the transcription of chiA (Figure 3; Table 1). Sequencing of the 310 bp LA-PCR2 fragment (Figure 3) located the TSP to base 54 of the S91 chiA sequence (AF007894); this was the same as TSP A1 found previously that possesses a putative σ70-dependent promoter (Delpin and Goodman, 2009). It is possible that there may be more than one regulatory system operating on S91 chiA. As the majority of genes possessing σ54-dependent promoters are involved in nitrogen metabolism (Reitzer and Schneider, 2001), the presence of the putative σ70-dependent promoter identified for TSP A1 (Delpin and Goodman, 2009) indicates that chiA may be controlled by another, or multiple, different regulatory system(s) (McGowan et al., 2003). For S91 grown in MMM glt, LA-PCR2 generated three fragments corresponding to A1, A2 and A3 (data not shown) indicating usage of all three TSPs. The presence of putative σ70- and σ54-dependent promoters for S91 chiA (Delpin and Goodman, 2009) suggests that there is complex and tight regulation of transcription (McGowan et al., 2003).
TAE agarose gel (3%) of LA-PCR2 reaction products (5 μl) of S91 chiA amplified from cDNA produced from RNA isolated from cells grown in marine minimal medium (MMM) maltose. Lane 1, 5 μl of a 50 ng μl−1 100 bp DNA ladder, 100 bp increments from 1000 to 100 bp; lane 2, MMM maltose; lane 3, reverse transcription (RT)-PCR-negative control of MMM maltose; lane 4, LA-PCR1-negative control; lane 5, LA-PCR2-negative control.
Conclusions and future directions
The major chitinase gene of Pseudoalteromonas sp. S91, chiA, appears to be part of the bacterium's nitrogen regulatory system, potentially being involved in nitrogen metabolism. chiA activity was induced by ammonium limitation and repressed by high ammonium concentrations. Work is underway to determine the promoter activity and TSP usage for chiA expression in S91 grown under different environmental conditions, for example during growth on natural complex substrates such as squid pen and under nitrogen starvation. In addition, the effects of nitrogen on TSP usage for chiB and chiC are being investigated. It is not known why chiA should use two σ54-dependent promoters. It is possible that there are further regulatory systems operating on the S91 chitinase genes and that there may be cross talk between them; preliminary data indicate that TSP A2 is the sole TSP used by chiA in S91 cells grown under oxygen-limited conditions.
Although many chitinase genes have been identified, cloned and sequenced, their regulation is still poorly understood. Most studies on the regulation of bacterial chitinases have focused on chitin as a carbon source, and the effects of various carbon substrates on gene expression. In this study nitrogen concentration and source was found to regulate promoter activity and use for the transcription of S91 chiA. It would be useful to determine the effect of nitrogen source and concentration, in conjunction with other environmental parameters, on chitinase gene expression in other bacterial species.
Accession codes
Abbreviations
- bp:
-
base pair
- GlcNAc:
-
N-acetylglucosamine
- glt:
-
glutamate
- LA-PCR:
-
ligation-anchored PCR
- MMM:
-
marine minimal medium
- MMM 20N:
-
marine minimal medium with 20 times the standard concentration of NH4Cl
- MMM NN:
-
marine minimal medium with no added NH4Cl
- sp. act:
-
β-galactosidase-specific activity
- TSP:
-
transcriptional start point
- TSPs:
-
transcriptional start points
- wt:
-
wild type
References
Albertson NH, Stretton S, Pongpattanakitshote S, Östling J, Marshall KC, Goodman AE et al. (1996). Construction and use of a new vector/transposon, pLBT::mini-Tn10:lac:kan, to identify environmentally responsive genes in a marine bacterium. FEMS Microbiol Lett 140: 287–294.
Atkinson MR, Blauwkamp TA, Bondarenko V, Studitsky V, Ninfa AJ . (2002). Activation of the glnA, glnK and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation. J Bacteriol 184: 5358–5363.
Buck M, Miller S, Drummond M, Dixon R . (1986). Upstream activator sequences are present in the promoters of nitrogen fixation genes. Nature 320: 374–378.
Cottrell MT, Kirchman DL . (2000). Natural assemblages of marine proteobacteria and members of the Cytophaga–Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66: 1692–1697.
Davalos M, Fourment J, Lucas A, Bergès H, Kahn D . (2004). Nitrogen regulation in Sinorhizobium meliloti probed with whole genome arrays. FEMS Microbiol Lett 241: 33–40.
Delpin MW, Goodman AE . (2009). Nutrient regime regulates complex transcriptional start site usage within a Pseudoalteromonas chitinase gene cluster. ISME J (in press).
Gooday GW . (1990). The ecology of chitin degradation. In: Marshall KC (ed). Advances in Microbial Ecology, vol. 11, Plenum Press: New York. pp 387–430.
Huang X, Miller W . (1991). A time-efficient, linear-space local similarity algorithm. Adv Appl Math 12: 337–357.
Keyhani NO, Roseman S . (1999). Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta 1473: 108–122.
Magasanik B . (1996). Regulation of nitrogen utilization. In: Neidhardt FC (ed). Escherichia coli and Salmonella Cellular and Molecular Biology, vol 1, ASM Press: Washington. pp 1344–1356.
McGowan CC, Necheva AS, Forsyth MH, Cover TL, Blaser MJ . (2003). Promoter analysis of Helicobacter pylori genes with enhanced expression at low pH. Mol Microbiol 48: 1225–1239.
Miller JH . (1972). Experiments in Molecular Genetics. Cold Spring Harbor: New York.
Östling J, Goodman AE, Kjelleberg S . (1991). Behaviour of InP-1 plasmids and a miniMu transposon in a marine Vibrio sp. S14: isolation of starvation inducible lac operon fusions. FEMS Microb Ecol 86: 83–94.
Reitzer LJ, Magasanik B . (1986). Transcription of glnA in Escherichia coli is stimulated by an activator bound to sites far from the promoter. Cell 45: 785–792.
Reitzer L, Schneider BL . (2001). Metabolic context and possible physiological themes of σ54-dependent genes in Escherichia coli. Microbiol Mol Biol R 65: 422–444.
Schneider BL, Shiau S-P, Reitzer LJ . (1991). Role of multiple environmental stimuli in control of transcription from a nitrogen-regulated promoter in Escherichia coli with weak or no activator-binding sites. J Bacteriol 173: 6355–6363.
Stretton S, Marshall KC, Dawes IW, Goodman AE . (1996). Characterisation of carbon dioxide-inducible genes of the marine bacterium, Pseudomonas sp. S91. FEMS Microbiol Lett 140: 37–42.
Techkarnjanaruk S, Pongpattanakitshote S, Goodman AE . (1997). Use of a promoterless lacZ gene insertion to investigate chitinase gene expression in the marine bacterium Pseudoalteromonas sp. S9. Appl Environ Microbiol 63: 2989–2996.
Tillett D, Burns BP, Neilan BA . (2000). Optimized rapid amplification of cDNA ends (RACE) for mapping bacterial mRNA transcripts. Biotechniques 28: 448–456.
Wetzel RG . (1991). Extracellular enzymatic interactions: storage, redistribution, and interspecific communication. In: Chrost RJ (ed). Microbial Enzymes in Aquatic Environments. Springer-Verlag: New York. pp 6–26.
Zar JH . (1996). Biostatistical Analysis. Prentice-Hall: New Jersey.
Zehr JP, Ward BB . (2002). Nitrogen cycling in the ocean: new perspectives on processes and paradigms. Appl Environ Microbiol 68: 1015–1024.
Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ et al. (2000). Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci USA 97: 14674–14679.
Acknowledgements
This work was supported in part by Flinders University and the Australian Research Council. Marina Delpin was supported by an Australian Postgraduate Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delpin, M., Goodman, A. Nitrogen regulates chitinase gene expression in a marine bacterium. ISME J 3, 1064–1069 (2009). https://doi.org/10.1038/ismej.2009.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2009.49
Keywords
This article is cited by
-
Characterization of regulatory regions involved in the inducible expression of chiB in Bacillus thuringiensis
Archives of Microbiology (2015)
-
Microdiversity of extracellular enzyme genes among sequenced prokaryotic genomes
The ISME Journal (2013)
-
Defense responses of soybean roots during exposure to cadmium, excess of nitrogen supply and combinations of these stressors
Molecular Biology Reports (2012)