Abstract
The conditions promoting the persistence of a plasmid carrying a trait that may be mutually beneficial to other cells in its vicinity were studied in structured and unstructured environments. A large plasmid encoding mercury resistance in Pseudomonas fluorescens was used, and the mercury concentration allowing invasion from rare for both plasmid-bearing and plasmid-free cells was determined for different initial inoculum densities in batch-culture structured (filter surface) and unstructured (mixed broth) environments. A range of mercury concentrations were found where both cell types could coexist, the regions being relatively similar in the two types of environment although density-dependent in the unstructured environment. The coexistence is explained in terms of frequency-dependent selection of the mutually beneficial mercury resistance trait, and the dynamics of bacterial growth under batch culture conditions. However, the region of coexistence was complicated by conjugation which increased plasmid spread in the mixed broth culture but not the structured environment.
Similar content being viewed by others
Main
Understanding plasmid persistence in bacterial populations is a major challenge for genetics and evolutionary biology (Bergstrom et al., 2000). The argument that most plasmids are parasitic and persist despite being costly to their hosts requires rates of horizontal transmission that are higher than normally observed (Turner et al., 1998; Bergstrom et al., 2000). Many plasmids harbour transiently beneficial traits, such as antibiotic resistance or the ability to utilize novel substrates (Eberhard, 1990; Sørensen et al., 2005), raising the question of why the advantageous gene is not captured by the host chromosome. Possibilities are that plasmid mobility allows the gene to be associated with selective sweeps through a population within an ecological niche, or because it allows gene flow among bacteria in different niches (Bergstrom et al., 2000; Thomas, 2004).
Plasmid persistence may also be influenced by frequency-dependent interactions. Many plasmid-borne gene products alter the external environment, benefiting neighbouring cells in addition to the host cell (Eberhard, 1990) and can be considered mutually beneficial (West et al., 2006) so that the relative fitness of plasmid-bearing and plasmid-free cells is frequency-dependent (Levin, 1988). Although they may have an advantage when rare, when plasmid-bearing cells are common, the plasmid-free cells are favoured as they benefit from the public good without paying the cost of plasmid carriage (Levin, 1988). A further complication is that plasmids may be able to infect neighbouring cells that have benefited from plasmid-borne genes (Smith, 2001). The extent to which these frequency-dependent interactions occur may be influenced by the spatial structure of the population (Nowak and Sigmund, 2004).
The frequency-dependent selection of a plasmid carrying a trait that modifies the local environment was explored using a mercury-resistance plasmid in Pseudomonas fluorescens SBW25 (Lilley and Bailey, 1997). When the resistance operon of pQBR103 is expressed, Hg2+ is imported into the plasmid-bearing cell by periplasmic scavenging enzymes and converted to the less toxic and volatile Hg0, thereby detoxifying the media (Silver and Misra, 1988). The coexistence of plasmid-free and plasmid-bearing cells was investigated while manipulating mercury chloride concentration, initial inoculum size and spatial structure (shaken broth culture or static filter culture). For each combination of conditions, pairs of experiments were initiated with the initial density of either plasmid-free or plasmid-bearing cells at approximately 10% to allow estimation of the likelihood of invasion when rare. Bacterial numbers reached their maxima after 3–4 days, when the relative densities of plasmid-bearing and plasmid-free cells were estimated by plate counting. Further experimental details can be found in the online Supplementary Information.
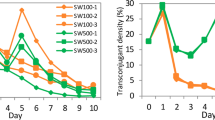
The results confirmed that plasmid-bearing cells have a selective advantage at high relative mercury toxicity and that plasmid-free cells have an advantage at low relative mercury toxicity. However, there was not a simple relative mercury toxicity threshold above which plasmid-carrying cells replace plasmid-free cells. In Figure 1, the relative toxicity threshold for the increase in frequency of plasmid-bearing cells does not coincide with the threshold for plasmid-free cells; there is a region between the red and green lines where both cell types can increase from rare, implying their coexistence in both structured and unstructured environments. These results can be explained in terms of the temporal dynamics of bacterial growth and mercury removal. There is a relative mercury toxicity threshold above which plasmid-free cells are inhibited but not killed, and above this threshold plasmid-bearing cells can grow while removing mercury from the system. If the initial mercury concentration is not too great, it will in time drop below the inhibitory threshold and therefore, providing sufficient resources remain, plasmid-free cells can begin to replicate (at a higher rate than plasmid-bearing cells as they not burdened by the plasmid) allowing both types to persist. Whether this occurs depends on the density of plasmid-bearing cells. In the mixed broth culture, the total density of plasmid-bearing cells is most important, whereas in the structured environment it is the local density of plasmid-bearing cells that will determine of the fate of neighbouring plasmid-free cells. In Figure 1, this is illustrated by the positive effect of initial density on the relative toxicity at which coexistence occurs in the mixed cultures, compared to the relatively weak effect of initial total density in the structured environment.
Relative mercury toxicity at which cell types can invade from rare at a range of starting densities in spatially structured environment (filters – solid lines) or homogeneous environment (broth – dashed lines) as a function of initial cell density. The green lines indicate minimum initial toxicity at which GFP (Hgr) cells can increase in frequency from 10%. The red lines indicate maximum initial toxicity at which RFP (Hgs) cells can increase in frequency from 10%. The area between the red and green lines is the conditions under which coexistence occurs. For the broth data, the effect of conjugation on the invasion of the plasmid is indicated by the orange line. The coexistence then occurs between the red and orange lines. Mercury concentrations have been converted to relative toxicity values as described in the Online Supplementary Information to facilitate direct comparison between structured and homogeneous environments.
The bacterium and plasmid were marked with genes encoding fluorescent proteins allowing the identification of transconjugants and the visualization of the spatial structure of plasmid-free and plasmid-bearing cells in the filter culture (Mølbak et al., 2007). Plasmid-free and plasmid-bearing cells tended to form discrete microcolonies, limiting physical contact and the opportunity for conjugation (Normander et al., 1998) until most of the space is occupied (Figure 2). This led to high local density of plasmid-bearing cells, which rapidly caused the local removal of mercury, thereby reducing toxicity and allowing the growth of plasmid-free cells. Although the spatial arrangement of microcolonies clearly differs between treatments (Figure 2), suggesting different spatial dynamics, it appears to have little effect on the conditions for coexistence indicated in Figure 1. No transconjugants were detected from the spatially structured habitat, but in the well-mixed environment horizontal transfer occurred allowing the plasmid to spread (orange line, Figure 1) at mercury concentrations below the threshold for invasion by plasmid-bearing cells in the absence of conjugation (red line, Figure 1). Although it is commonly thought that conjugation is more efficient on surfaces than in liquid, it is dependent on the initial density of cells (Lilley and Bailey, 2002; Lagido et al., 2003) and therefore the low initial cell density used here explains the lack of transconjugants observed in the spatially structured habitat. In the mixed environment, there is a much higher likelihood of plasmid-bearing and plasmid-free cells encountering one another in and thus for conjugation to occur.
Epifluorescence images (captured as described in the Online Supplementary Information) of spatial arrangement of plasmid-bearing GFP (Hgr) and plasmid-free RFP (Hgs) microcolonies at densities and mercury toxicity within the area of coexistence indicated in Figure 1. Horizontal pairs of images are of GFP (Hgr) and RFP (Hgs) cells within the same field of view. Groups of four images illustrate the outcomes when one or other of the cell types was initially rare, but at the same mercury concentration. Start and end ratios have been determined by plate counts (see Online Supplementary Information). Each field of view is approximately 1 mm2.
This study explored the coexistence of plasmid-bearing and plasmid-free cells in spatially structured and well-mixed environments. Coexistence occurs because of frequency-dependent selection on a trait than can also benefit non-carrier cells, and the temporal dynamics of the batch cultures experiments. Although the experimental design is artificial, Pseudomonas spp. inhabiting the phytosphere are likely to exhibit batch-type growth dynamics, whereas their plasmids are known to carry genes for other mutually beneficial traits in addition to mercury resistance. The generality of the explanation provides a suitable framework for understanding the persistence of plasmids in many systems, but the current challenge is to explore the dynamics of plasmids in more realistic natural environments.
References
Bergstrom CT, Lipsitch M, Levin BR . (2000). Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genet 155: 1505–1519.
Eberhard WG . (1990). Evolution in bacterial plasmids and levels of selection. Q Rev Biol 65: 3–22.
Lagido C, Wilson IJ, Glover LA, Prosser JI . (2003). A model for bacterial conjugal gene transfer on solid surfaces. FEMS Microbiol Ecol 44: 67–78.
Levin BR . (1988). Frequency-dependent selection in bacterial populations. Phil Trans R Soc Lond B 319: 459–472.
Lilley AK, Bailey MJ . (1997). Impact of plasmid pQBR103 acquisition and carriage on the phytosphere fitness of Pseudomonas fluorescens SBW25: burden and benefit. Appl Environ Microbiol 63: 1584–1587.
Lilley AK, Bailey MJ . (2002). The transfer dynamics of Pseudomonas sp. plasmid pQBR11 in biofilms. FEMS Microbiol Ecol 42: 243–250.
Mølbak L, Molin S, Kroer N . (2007). Root growth and exudate production define the frequency of horizontal plasmid transfer in the rhizosphere. FEMS Microbiol Ecol 59: 167–176.
Normander B, Christensen BB, Molin S, Kroer N . (1998). Effect of bacterial distribution and activity on conjugal gene transfer on the phylloplane of the bush bean (Phaseolus vulgaris). Appl Environ Microbiol 64: 1902–1909.
Nowak MA, Sigmund K . (2004). Evolutionary dynamics of biological games. Science 303: 793–799.
Silver S, Misra TK . (1988). Plasmid-mediated heavy metal resistances. Annu Rev Microbiol 42: 717–743.
Smith J . (2001). The social evolution of bacterial pathogenesis. Proc R Soc Lond B 268: 61–69.
Sørensen S, Bailey MJ, Hansen LH, Kroer N, Wuertz S . (2005). Studying plasmid horizontal transfer in situ: a critical review. Nat Rev Microbiol 3: 700–710.
Thomas CM . (2004). Evolution and population genetics of bacterial plasmids. In: Funnell BE and Phillips GJ (eds). Plasmid Biology. ASM Press: Washington DC, pp. 509–528.
Turner PE, Cooper VS, Lenski RE . (1998). Tradeoff between horizontal and vertical modes of transmission in bacterial plasmids. Evolution 52: 315–329.
West SA, Griffin AS, Gardner A, Diggle SP . (2006). Social evolution theory for microorganisms. Nat Rev Microbiol 4: 597–607.
Acknowledgements
We are grateful to the UK Natural Environment Research Council for their funding of this work. We also thank Tracey Timms-Wilson for the kind gift of Pseudomonas fluorescens SBW25R∷RFP and Kim Prior for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej).
Supplementary information
Rights and permissions
About this article
Cite this article
Ellis, R., Lilley, A., Lacey, S. et al. Frequency-dependent advantages of plasmid carriage by Pseudomonas in homogeneous and spatially structured environments. ISME J 1, 92–95 (2007). https://doi.org/10.1038/ismej.2007.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2007.11
Keywords
This article is cited by
-
Negative frequency dependent selection on plasmid carriage and low fitness costs maintain extended spectrum β-lactamases in Escherichia coli
Scientific Reports (2019)
-
Biofilms facilitate cheating and social exploitation of β-lactam resistance in Escherichia coli
npj Biofilms and Microbiomes (2019)
-
Emergence of nontoxic mutants as revealed by single filament analysis in bloom-forming cyanobacteria of the genus Planktothrix
BMC Microbiology (2016)
-
Live to cheat another day: bacterial dormancy facilitates the social exploitation of β-lactamases
The ISME Journal (2016)
-
Evolving Concepts of Bacterial Species
Evolutionary Biology (2012)