Abstract
This paper aims to investigate if the dental restoration of nickel–chromium based alloy (Ni–Cr) leads to the enhanced excretions of Ni and Cr in urine. Seven hundred and ninety-five patients in a dental hospital had single or multiple Ni–Cr alloy restoration recently and 198 controls were recruited to collect information on dental restoration by questionnaire and clinical examination. Urinary concentrations of Ni and Cr from each subject were measure by graphite furnace atomic absorption spectrometry. Compared to the control group, the urinary level of Ni was significantly higher in the patient group of <1 month of the restoration duration, among which higher Ni excretions were found in those with either a higher number of teeth replaced by dental alloys or a higher index of metal crown not covered with the porcelain. Urinary levels of Cr were significantly higher in the three patient groups of <1, 1 to <3 and 3 to <6 months, especially in those with a higher metal crown exposure index. Linear curve estimations showed better relationships between urinary Ni and Cr in patients within 6-month groups. Our data suggested significant increased excretions of urinary Ni and Cr after dental restoration. Potential short- and long-term effects of Ni–Cr alloy restoration need to be investigated.
Similar content being viewed by others
Introduction
Dental alloy restorations are widely used for fixed prosthodontics. Cast gold alloy and ceramic materials are the most widely used restorations in the developed countries, while base metal alloys and stainless-steel crowns are commonly used in the developing countries.1 Metal casting alloys, especially nickel (Ni) based alloys, have raised public health concerns for their biocompatibility in the oral cavity. The term biocompatibility in some aspects refers to the elemental ion release of toxic metals from casting alloys, which is suspected to lead to some adverse biological effects, such as cytotoxicity,2,3 genotoxicity4 and allergy.5,6
A number of studies have investigated the amount of elemental ion release from dental alloys both in vitro2,7,2,8,9,10,11,12,13,14 and in vivo.15,16,17 Ni-based alloys often use nickel–chromium (Ni–Cr) based materials which contain Ni between 60 % and 84 % (m/m), and Cr between 8 % and 26 % (m/m).7 Release of both Ni and Cr from Ni–Cr alloy under simulated oral conditions has been well described in vitro.15,18,9,10 However, there is lack of toxicological and epidemiologic data on Ni release in vivo.15
Nickel is a known allergen. It has been reported that the prevalence of Ni hypersensitivity is 8%–28% in females and 1%–5% in males in Scandinavian populations.18 There has been no prevalence data so far in China, but it has been found that Ni hypersensitivity is the leading cause of contact dermatitis based on patch tests.19 The ion release of Ni in the oral cavity is one of the suspected Ni exposures in the environment. There were few case reports of allergic reactions to intraoral nickel.6,20 Sweden’s National Board of Health and Welfare has advised that no dental material containing over 1% Ni should be placed into a patient’s mouth.21
Long-term exposure to low levels of Ni and Cr may result in an accumulation in the kidney and lead to renal dysfunction.22 In March 2009 in China, a man 31 years of age claimed on the media that his 4-year history of albuminuria and hematuria was probably associated with the Ni–Cr alloy restoration placed 4 years ago, which caused a public outcry.23 Although there is no clear evidence for nephrotoxicity of dental alloy, this event dramatically discouraged patients to have Ni–Cr alloys. Therefore, there is an urgency to clarify the issues and the current study examined the associations of Ni–Cr alloy restoration with elemental ion release and renal dysfunction.
Materials and methods
Subjects
This study was conducted in the largest dental hospital and its county branches in Shanghai between April and September, 2009. We contacted over 2000 former patients who previously had Ni–Cr alloy restoration, and invited them to participate in the current study. Eight hundred and three patients visited the hospital after the invitation (patient group). For comparison, we also recruited 85 visiting outpatients and 113 hospital employees who had no history of alloy restoration (control group). None of the study subjects had orthodontic therapy and occupational exposure to Ni and Cr.
The participants completed a self-administered questionnaire which covered the information on sex, age, height, weight, smoking, alcohol consumption and the dental restoration by metal alloy. Information on the dental restoration by alloy for the patient group included times of alloy therapies and when and where each therapy was conducted and how many teeth were replaced by alloy. A clinical examination was conducted to determine if the metal crown was fused to the porcelain. This fusion, in some cases, was not performed very well in technique and would lead to the direct exposure of metal surface to the saliva environment and therefore was hypothesized to increase the ion release. The surface area of metal crown uncovered with porcelain was categorized into three groups: (i) less than 1/5 surface of one tooth; (ii) 1/5 to 1/2 and (iii) higher than 1/2. An index of metal crown not covered with the fused porcelain was measured by multiplying the surface area (categorized group of 1, 2 or 3) and the number of teeth replaced by Ni–Cr alloys.
Determination of Ni and Cr
Subjects provided urine and serum specimens for the measurements of Ni and Cr levels as well as evaluating the renal function. Eight patients did not provide serum samples, and this analysis was based on data from 795 patients 198 controls who had complete information.
Spot urine sample of 50 mL was collected from each individual using a polyethylene vessel which was soaked overnight in 3% nitric acid and washed in clean water to confirm no background pollution of heavy metals. After arrival at the laboratory, the samples were acidified in 1% nitric acid except for those for creatinine measurements. Both urine and serum samples were stored at −80 °C in polyethylene eppendorf tubes.
The levels of Ni and Cr in urine were measured by graphite furnace atomic absorption spectrometry with Zeeman background correction (Perkin Elmer AAnalyst 800 spectrometer, Perkin Elmer, Waltham, MA, USA). Determination of Ni was performed according to the standard method of WS/T 44-1996 recommended by the China Ministry of Health at the wavelength of 232.2 nm (WS/T 44-1996).24 Determination of Cr was performed according to the standard method of WS/T 37-1996 at the wavelength of 357.9 nm (WS/T 37-1996).25 Calibration was performed with six samples of spiked natural urine in the range of 0–250 µg⋅L−1 Ni or Cr. For internal quality control, spiked urine sample was measured repeatedly every set of 20 samples, and the interday relative standard deviation was limited to <0.5% and the intraday relative standard deviation <1%. The limits of determination (LOD) were 1 µg⋅L−1 for both Ni and Cr in urine. Values below the LOD were set to 1/2 of the LOD. The recoveries were 80%–105.7% for Ni and 95.8%–108.3% for Cr.
Statistical analyses
Urinary concentrations of Ni and Cr were ln-transformed. Student’s t test, one-way analysis of variance were used to test differences in Ni and Cr concentrations between the patient and control groups, and the linear curve estimation was used to examine the association between Ni–Cr alloy restoration and extra excretion of Ni and Cr in urine. The covariates were set as follows: sex (male, female), age (<40, 40–52, 53–58 and ≥59 years), body mass index (<24.0, 24.0–27.9 and ≥28.0 kg·m−2), cigarette smoking (non-smoker, smoker) and alcohol drinking (non-drinker, drinker). All pooled subjects were quartered at equal sample size by the age variable, and this classification was used to compare the age difference between the patients and controls. The classification of body mass index was according to the recommendation of the Working Group on Obesity in China.26 All statistic tests were two sided (α=0.05) and performed by using SPSS10.0.
Results
Table 1 showed the comparisons between study characteristics. The patients were older, and were more likely to be a female or a non-smoker compared with controls.
According to the duration of Ni–Cr alloy restoration, patients were further grouped into five groups: <1 month (205, 26%), 1 to <3 months (128, 16%), 3 to <6 months (130, 16%), 6 to <12 months (152, 19%) and ≥12 months (180, 23%). Over half of the patients (449, 56%) had three or more teeth replaced by Ni–Cr alloys. Only 133 (17%) patients had the alloy fused well to the porcelain, over 1/3 (300, 38%) patients had metal crowns badly fused to the porcelain (index level of ≥3), the others (362, 46%) had some crowns not covered with the porcelain (index level of 1 or 2).
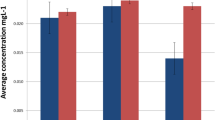
Figure 1 showed the urinary concentrations of Ni and Cr in association with the duration of alloy restoration. A significant increased level of Ni in urine was only found in patient group of <1 month (87.75 µg⋅mol−1 creatinine) compared to the control group (54.48 µg⋅mol−1 creatinine) (P<0.001). Significant increased levels of urinary Cr were found in three patient groups of <1 month (63.80 µg⋅mol−1 creatinine), 1 to <3 months (62.93 µg⋅mol−1 creatinine) and 3 to <6 months (57.62 µg⋅mol−1 creatinine) compared to the control group (43.83 µg⋅mol−1 creatinine) (P<0.05).
Figure 2 shows the levels of urinary Ni and Cr in association with the number of tooth replaced by Ni–Cr alloy and the index of metal crown not covered with the porcelain. In the patient group of <1 month of the restoration duration (n=205), Ni excretions in urine were significantly higher in those who had more teeth replaced by the Ni–Cr alloys (≥3: 103.20 µg⋅mol−1 creatinine; <3: 70.73 µg⋅mol−1 creatinine; P=0.019) or in those who had higher index of metal crown not covered with the porcelain (≥3: 118.37 µg⋅mol−1 creatinine; <3: 73.02 µg⋅mol−1 creatinine; P=0.012). In the pooled patient group of <6 months of restoration duration (<1, 1 to <3 and 3 to <6 months, total number of patients was 463), Cr excretions in urine were significantly higher in those who had higher index of metal crown not covered with the porcelain (≥3: 74.96 µg⋅mol−1 creatinine; <3: 56.08 µg⋅mol−1 creatinine; P=0.012).
Urinary levels of Ni and Cr in association with the number of teeth replaced by Ni–Cr alloys and the index of metal crown not covered with the porcelain. Ni excretions in urine were only analyzed in the patient group of <1 month of the restoration duration (n=205), and Cr excretions only in the pooled patient group of <6 months of restoration duration (n=463). Data were shown as geometric mean and 25%–75% interquartile range. Cr, chromium; Ni, nickel.
Figure 3 presents linear curve estimations for the relationship between urinary Ni and Cr. There were stronger relationships between Ni and Cr concentrations in those patient groups of <6 months comparing to the other patient groups or the control group. The Ni–Cr correlation was most pronounced in the patient group of <1 month, although the value of R2 was only 0.101 (slope: 0.37, P<0.001).
Association between ln-transformed urinary concentrations of Ni and Cr. In the subjects <1 month, which had the highest R square (R2) of 0.101 and the most significant of P value (P<0.001). The urinary Ni-Cr associations in the subjects from other duration groups had much lower values of R2 (R2 = 0.064, 0.065, 0.024, <0.001, for the duration group of 1 to <3, 3 to <6, 6 to <12, and ≥12, respectively). Cr, chromium; Ni, nickel.
Discussion
Our data demonstrated that there were enhanced urine excretions of Ni and Cr after Ni–Cr alloy restoration. The release of metal ions from dental alloys has been demonstrated in vitro.2,7,8,9,10,11,12,13,14,27 A significant Ni release has been shown in both cell culture system12,14 and artificial saliva system,7,8 and can cause cellular function damage and the decrease of cell viability in fibroblasts.2,28 The amount of Ni release is generally higher for Ni–Cr alloys compared with that for other alloys.1,8 Cr release has also been reported in association with Ni–Cr alloy, but in a less degree.8,13
Although ion release from dental materials is evident in vitro, study results are rare and conflicting in vivo.15,16,17 After replacement of orthodontic appliances, Bishara et al.17 did not found any significant change in serum Ni and Cr in 31 patients in 2 or 4–5 months later, while Menezes et al.16 reported a significantly increased urinary level of Ni in 21 patients 2 months later. For Ni-based restorative alloys, patients undergoing Ni–Cr replacement 1 year ago had a higher level of serum Ni than that of controls.15 Other Ni-based implants have also demonstrated significantly increased Ni concentrations in body fluids after the implantation, such as hip prostheses,29 cardiac device,30 spinal instrumentation31 and tungsten alloy.32
People may believe that the amount of Ni release from dental alloys is likely to be less as compared with that from dietary intake. There are no data of daily dietary intake of Ni in China. Ni intake from the Danish diet was estimated to be 150 µg·d−1 on average,33 and that from the England diet to be 1.49–1.63 µg·kg−1·d−1 in adults,34 which tend to be higher than the amount of Ni or Cr release from dental alloys in most in vitro studies. Wataha et al.14 reported that the total amount of Ni release from a Ni–Cr alloy was among the lowest at about 6 µg⋅cm−2 after immersing different casting alloys into cell culture medium over 10 months. However, Ni release in the cell culture medium may underrate the situation, as also by Wataha et al.,12 Ni–Cr alloy released above 22 µg⋅cm−2 of Ni in 0.85% sodium chloride (NaCl) solution for 7 days immersion, but less than 1 µg⋅cm−2 in culture medium. Also in sodium chloride solution, Elshahawy et al.1 reported even higher Ni release of 220 µg⋅cm−2for 7 days from Ni–Cr alloy. Ni release from artificial saliva was similar to that from saline solution, as Lopez-Alias et al.8 reported that a beryllium-containing Ni-based alloy released 5.22 µg⋅cm−2·d−1 of Ni. This rate of ion release, according to Lopez-Alias et al., would lead to 50% of the upper tolerable intake level (1 000 µg·d−1) in the worst scenario in which a subject had all 32 teeth covered by full metal crowns.8 Therefore, Ni exposure from Ni–Cr alloy might be at the same level of dietary intake or even more, which could lead to an enhanced excretion of urinary Ni.
Our data also showed an enhanced Cr excretion in urine, suggesting that Cr release from dental restoration might be an important exposure source. Previous studies have shown that Cr release from Ni–Cr alloy is much lower than Ni release.1,8 One study found that Ni release was 220 µg⋅cm−2 after 7 days immersion into the saline solution, but Cr release was only 0.2 µg⋅cm−2 (ref. 1). Another study demonstrated that Cr release was about half of the amount of Ni release when immersing orthodontic appliances into acidiferous water.13 The amount of ion release is likely related to the compositions of different metals. In our study, although all patients had Ni–Cr alloys, there was a lack of detail information on metal compositions.
Another important finding of our study is that both Ni and Cr excretions in urine were temporarily associated with dental restoration. This might be explained by temporary releases of Ni and Cr from the alloys, which had been demonstrated in some in vitro26 and in vivo studies.35 Barrett et al26 demonstrate in an in vitro study that Ni release from orthodontic appliances of nickel–titanium (Ni–Ti) and stainless steel increased over the first week then diminished over time, and that Cr release increased during the first 2 weeks and leveled off in the subsequent 2 weeks. Sample saliva from patients fitted with fixed orthodontic appliances revealed a significant initial increase in Ni release within the first three weeks then diminished over time.34 Similar results were obtained in an investigation of 50 patients wearing Ni–Cr or Ni–Cr–Ti alloys, with increased but not significant concentrations of Ni at the first week but went back in 3 months or 6 months.36 The prolonged Ni or Cr release in vivo than in vitro might be related to the sanitary habits such as tooth brushing and mouth rinsing.
One more detail that should also be noted is that the significant association between Ni and Cr concentrations with 1 month as shown in Figure 3 had low value of R square, which althought became the highest value than those R sequares from the associations in other time groups. This might indicate two facts. One is that the co-exposure to Ni and Cr after the dental restoration could be at a relatively low level. The other is that both Ni and Cr excretions in urine might have other explanations such as dietary exposure. However, this low level of co-exposure can not be beyond a reasonable doubt that Ni and Cr exposure from dental restoration should be concerned. As mentioned above, the release of Ni and Cr after the alloy replacement could be happened at different times. Cr release was more prolonged that the Ni release. The low level of co-exposure might simply indicate that the asynchronous release.
The present study had several shortcomings. Firstly, we did not investigate the dietary intake of Ni or Cr from participants. However, we could assume that patients and controls were comparable. Secondly, frying ferrum or Ni pan is commonly used in Chinese families, but most participants were not aware the types of their frying pans. Thirdly, we had no information on the compositions of Ni and Cr for alloys that patients wear.
The biological effect of a temporary systemic increase in Ni and Cr levels in human is unknown. Although literature reported some cases of Ni hypersentivity in patients undergoing Ni–Cr restoration, only few cases in this study reported black lines on the gums (13 cases) or pulpitis (1 case). It should also be noticed that even if Ni–Cr alloy restoration will lead to the extra exposure to Ni and Cr, the levels are not sufficient to cause alarm (the geometric means of urinary Ni and Cr in this study were lower than some reports in China37,38). However, further studies need to confirm our findings and determinate the implications of these findings.
Conclusions
This study investigated whether urinary levels of Ni and Cr were associated with the dental restoration of Ni–Cr alloys. The temporary increased level of Ni in urine was limited to within 1 month, and temporary increased level of Cr in urine was limited to within 6 months. These temporary increased levels of metals after Ni–Cr restoration were associated with the number of restorative alloys and the level of metal crown not covered with the porcelain. This is the first time according to our knowledge that a large sample size population investigation showed that the Ni–Cr alloy restoration may become an important source of Ni and Cr exposure. The potential short- and long-term effects of the Ni and Cr exposure after dental restoration need to be investigated.
References
Elshahawy W, Watanabe I, Koike M . Elemental ion release from four different fixed prosthodontic materials. Dent Mater 2009; 25( 8): 976–981.
Elshahawy WM, Watanabe I, Kramer P . In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent Mater 2009; 25( 12): 1551–1555.
Wylie CM, Davenport AJ, Cooper PR et al. Oral keratinocyte responses to nickel-based dental casting alloys in vitro. J Biomater Appl 2010; 25( 3): 251–267.
Tripuraneni SC, Namburi SK . Evaluation of genotoxicity of recycled Ni–Cr dental casting alloys: an in vitro study. J Appl Biomater Biomech 2008; 6( 1): 47–54.
Lu Y, Chen W, Ke W et al. Nickel-based (Ni–Cr and Ni–Cr–Be) alloys used in dental restorations may be a potential cause for immune-mediated hypersensitivity. Med Hypotheses 2009; 73( 5): 716–717.
Noble J, Ahing SI, Karaiskos NE et al. Nickel allergy and orthodontics, a review and report of two cases. Br Dent J 2008; 204( 6): 297–300.
Jones SB, Taylor RL, Colligon JS et al. Effect of element concentration on nickel release from dental alloys using a novel ion beam method. Dent Mater 2010; 26( 3): 249–256.
Lopez-Alias JF, Martinez-Gomis J, Anglada JM et al. Ion release from dental casting alloys as assessed by a continuous flow system: Nutritional and toxicological implications. Dent Mater 2006; 22( 9): 832–837.
Wylie CM, Shelton RM, Fleming GJ et al. Corrosion of nickel-based dental casting alloys. Dent Mater 2007; 23( 6): 714–723.
Denizoglu S, Duymus ZY, Akyalcin S . Evaluation of ion release from two base-metal alloys at various pH levels. J Int Med Res 2004; 32( 1): 33–38.
Baucic M, Celebic A, Stipetic J et al. In vitro release of metal ions from a gold–platinum alloy in saliva-simulated conditions. Coll Antropol 2003; 27 ( Suppl 2): 91–98.
Wataha JC, Nelson SK, Lockwood PE . Elemental release from dental casting alloys into biological media with and without protein. Dent Mater 2001; 17( 5): 409–414.
Staffolani N, Damiani F, Lilli C et al. Ion release from orthodontic appliances. J Dent 1999; 27( 6): 449–454.
Wataha JC, Lockwood PE . Release of elements from dental casting alloys into cell-culture medium over 10 months. Dent Mater 1998; 14( 2): 158–163.
Liu C, Li R, An F . Changes of serum nickel and chromium content at 6 months and 1 year after porcelain-fused-to-nichrome crown restoration: A comparison with healthy controls. J Clin Rehab Tissue Eng Res 2008; 12( 23): 4583–4585.
Menezes LM, Quintao CA, Bolognese AM . Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofacial Orthop 2007; 131( 5): 635–638.
Bishara SE, Barrett RD, Selim MI . Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofacial Orthop 1993; 103( 2): 115–119.
Schram SE, Warshaw EM, Laumann A . Nickel hypersensitivity: a clinical review and call to action. Int J Dermatol 2010; 49( 2): 115–125.
Yin R, Huang XY, Zhou XF et al. A retrospective study of patch tests in Chongqing, China from 2004 to 2009. Contact Dermatitis 2011; 65( 1): 28–33.
Kolokitha OE, Kaklamanos EG, Papadopoulos MA . Prevalence of nickel hypersensitivity in orthodontic patients: a meta-analysis. Am J Orthod Dentofacial Orthop 2008; 34( 6): 722 e1–e12; discussion 3.
Pierce LH, Goodkind RJ . A status report of possible risks of base metal alloys and their components. J Prosthet Dent 1989; 62( 2): 234–238.
Strauss FG, Eggleston DW . IgA nephropathy associated with dental nickel alloy sensitization. Am J Nephrol 1985; 5( 5): 395–397.
Oriental Horizon. Deep thinking trigged by porcelain fused metal crown. China Central Television 2009; 14 April 2009. http://news.xinhuanet.com/video/2009-04/14/content_11185134.htm ( accessed 20 June 2010).
China national standardization management committee. Health standard. Urine. Determination of nickel. Graphite furnace atomic absorption spectrometry method. WS/T 44-1996. Beijing: Standards Press of China, 1996. Chinese.
China national standardization management committee. Health standard. Urine. Determination of chromium. Graphite furnace atomic absorption spectrometry method. WS/T 37-1996. Beijing: Standards Press of China, 1996. Chinese.
The Working Group on Obesity in China (WGOC). The recommendation of body mass index cut off points for Chinese adult. Chin J Prev Med 2001; 35: 349–350.
Barrett RD, Bishara SE, Quinn JK . Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop 1993; 103( 1): 8–14.
Messer RL, Lucas LC . Cytotoxicity of nickel–chromium alloys: bulk alloys compared to multiple ion salt solutions. Dent Mater 2000; 16( 3): 207–212.
Sunderman FW Jr, Hopfer SM, Swift T et al. Cobalt, chromium, and nickel concentrations in body fluids of patients with porous-coated knee or hip prostheses. J Orthop Res 1989; 7( 3): 307–315.
Burian M, Neumann T, Weber M et al. Nickel release, a possible indicator for the duration of antiplatelet treatment, from a nickel cardiac device in vivo: a study in patients with atrial septal defects implanted with an Amplatzer occluder. Int J Clin Pharmacol Ther 2006; 44( 3): 107–112.
del Rio J, Beguiristain J, Duart J . Metal levels in corrosion of spinal implants. Eur Spine J 2007; 16( 7): 1055–1061.
Kalinich JF, Vergara VB, Emond CA . Urinary and serum metal levels as indicators of embedded tungsten alloy fragments. Mil Med 2008; 173( 8): 754–758.
Flyvholm MA, Nielsen GD, Andersen A . Nickel content of food and estimation of dietary intake. Z Lebensm Unters Forsch 1984; 179( 6): 427–431.
Rose M, Baxter M, Brereton N et al. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2010; 27( 10): 1380–1404.
Gjerdet NR, Erichsen ES, Remlo HE et al. Nickel and iron in saliva of patients with fixed orthodontic appliances. Acta Odontol Scand 1991; 49( 2): 73–78.
Wang WJ, Zhang TQ, Wei H . [Study on content of nickel in saliva released from the nickel–chromium and the nickel–chromium–titanium porcelain alloy.] Hua Xi Kou Qiang Yi Xue Za Zhi 2010; 28( 1): 45–47. Chinese.
Yang Y, Jin XM, Yan CH et al. Urinary level of nickel and acute leukaemia in Chinese children. Toxicol Ind Health 2008; 24( 9): 603–610.
Zhang XH, Zhang X, Wang XC et al. Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health 2011; 11: 224.
Acknowledgements
The study was undertaken with the permission of the local authority and the ethics committee of the School of Public Health, Fudan University. Informed consent was obtained from each participating individual. Publication of this manuscript is supported by Open Fund of State Key Laboratory of Oral Diseases, Sichun University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Chen, B., Xia, G., Cao, XM. et al. Urinary levels of nickel and chromium associated with dental restoration by nickel–chromium based alloys. Int J Oral Sci 5, 44–48 (2013). https://doi.org/10.1038/ijos.2013.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2013.13
Keywords
This article is cited by
-
Evaluation of the release of nickel and titanium under orthodontic treatment
Scientific Reports (2020)