Abstract
Bacterial infection associated with the use of medical or dental devices is a serious concern. Although devices made of ethylene vinyl acetate (EVA) are often used in the oral cavity, there are no established standards for their storage. We investigated bacterial survival on EVA sheets under various storage conditions to establish a standard for hygienic storage of such dental devices. Bacterial counts were evaluated, which showed a significant decrease after washing with sterilized water, mechanical brushing and rinsing, and using Mouthguard Cleaner as compared to untreated samples. In addition, no bacteria were detected on samples stored 2 days or longer in a ventilated environment, whereas they were detected for up to 14 days on samples without any cleaning stored in a closed environment. Bacterial counts for the untreated samples gradually declined, while surviving bacteria on samples treated with sterilized water and mechanical brushing showed a rapid decrease. Additionally, bacterial identification using polymerase chain reaction (PCR) revealed that Streptococcus oralis was dominantly detected on salivary samples after 14 days of storage among both two subjects. For effective hygienic storage of dental devices made of EVA, washing with sterilized water is important to remove absorbed salivary compounds along with storage in a ventilated environment.
Similar content being viewed by others
Introduction
Ethylene vinyl acetate (EVA) is an elastic thermoforming material frequently used in custom-made dental devices, including sports mouthguards, occlusal splints and dental drug delivery system trays.1,2 Mouthguards have been utilized by athletes who recognize the need for oral protection during their sports activities.3 Techniques that utilize dental drug delivery system trays have been developed for controlling oral cariogenic bacteria, in combination with professional mechanical tooth cleaning (PMTC), and they are designed for repetitive usage within 1 day for care at home performed by patients.2
According to their purpose and design, dental devices made of EVA are used repeatedly from 1 day to several months, and the method of storage of such devices is the responsibility of each individual user. However, no standard for adequate storage for EVA devices has been established. Thus, the storage methods utilized are considered to differ and may not be appropriate from the perspective of bacteriological safety.
Herein, we investigated bacterial survival on samples made of EVA under various storage conditions, and evaluated the effectiveness based on microbiological and other aspects. Our purpose is to provide data to establish a standard for hygienic storage for dental devices made of EVA.
Materials and methods
Subjects and sample collection
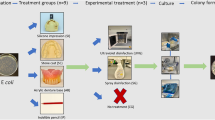
The study subjects consisted of seven healthy adults ranging from 24 to 32 years old, who belonged to the Department of Prosthodontics, Gerodontology and Oral Rehabilitation, Osaka University Graduate School of Dentistry. The study protocol was approved by the Ethics Board of the Institute of Dentistry, Osaka University, and informed consent was obtained from each subject. Prior to the collection of individual specimens, dental biofilm was removed using PMTC by a single trained dental hygienist. Following PMTC, the subjects rinsed their mouth with sterilized water. Next, an EVA sheet (Erkoflex; ERKODENT Erich Kopp GmbH, Pfalzgrafenweiler, Germany) measuring 5 mm×20 mm×1 mm was placed in the mouth to mix with saliva for 5 min. Salivary bacterial counts were determined after incubation of 100-fold diluted saliva on brain–heart infusion (BHI) agar plates. EVA sample sheets were washed with sterilized running water from wash bottles made up of polyethylene for 10 s, then subjected to mechanical brushing with a toothbrush 5 times and cetylpyridinium chloride solution (Mouthguard Cleaner; Earth Chemical Co. Ltd, Tokyo, Japan), either individually or in combination, then stored for 0, 1, 2, 3, 7, 14, 21 or 28 days in ventilated containers (Retainer Case; A. R. Medicom Inc. (Asia) Ltd, Kobe, Japan) or closed tubes (Eppendorf tube; Eppendorf Biochip Systems GmbH, Hamburg, Germany) at room temperature until performance of a detection assay.
Isolation and identification of oral bacteria on EVA sample sheets
Salivary samples obtained from the surfaces of the EVA sheets were serially diluted with 1.0 mL of sterilized phosphate-buffered saline (PBS), then inoculated onto BHI agar plates and incubated at 37 °C for 48 h in a 5% CO2 enriched atmosphere. At least 100 colonies were randomly selected from each sheet and identified.
Bacterial identification using polymerase chain reaction (PCR)
PCR was carried out in 20 µL of a reaction mixture containing 0.5 U Ampli Taq Gold DNA Polymerase (Life Technologies Japan, Ltd., Tokyo, Japan), 1.0 µmol⋅L−1 of oligonucleotide primers, and 1.5 mmol⋅L−1 of MgCl2, according to the manufacturers’ instructions. Amplification was performed with a GeneAmp 2400 thermal cycler (Applied Biosystems). Nine primer sets was used for the detection of oral streptococci, including Streptococcus mitis, Streptococcus salivarius, Streptococcus sanguinis, Streptococcus oralis, Streptococcus gordonii, Streptococcus mutans, Streptococcus sobrinus, Streptococcus pyogenes and Streptococcus pneumoniae, as previously described.4,5,6,7 The PCR products were stained with ethidium bromide and analyzed using 1.5% agarose gel electrophoresis.
Statistical analyses
The difference was calculated with logarithmic transformation of bacterial counts followed by comparison by using the Student’s t-test. Statistical significance was considered in case for P values less than 0.05.
Results
Detection of bacteria on EVA sheets
Following PMTC, the salivary bacterial count in one of the subjects was examined. EVA sample sheets mixed with saliva were strongly vortexed with sterilized PBS and inoculated onto BHI ager plates. The mean salivary bacterial count of the untreated sheets was (4.74±1.62)×106 colony forming units (CFU)⋅mL−1, which was significantly decreased by washing with sterilized water (P=0.031), mechanical brushing and rinsing (P=0.020), and using cetylpyridinium chloride (P<0.001) as shown in Figure 1.
Number of attached bacteria on EVA sheets with different cleaning methods. Following PMTC, salivary bacterial counts were determined for one of the subjects. EVA sample sheets mixed with saliva were strongly vortexed with 1 mL sterilized PBS, then inoculated onto BHI agar plates and incubated for 24 h. The mean number of salivary bacteria was (4.74±1.62)×106 CFU per 1 mL saliva. The sample sheets were treated with sterilized water (rinse), mechanical brushing and rinsing (brushing), cetylpyridinium chloride solution (Mouthguard Cleaner; MGC) and without any cleaning (untreated), respectively. The vertical axis shows bacterial counts per samples for solid surfaces, except for saliva. Statistical significance was determined using a Student’s t-test (*P<0.05, **P<0.001 vs. untreated control group). All experiments were performed in triplicate with three technical repeats. Horizontal bars indicate the standard deviation. BHI, brain–heart infusion; CFU, colony-forming units; EVA, ethylene vinyl acetate; PBS, phosphate-buffered saline; PMTC, professional mechanical tooth cleaning.
Next, EVA sample sheets mixed with saliva were strongly vortexed with sterilized PBS to correct bacteria from solid surface, and inoculated onto BHI ager plates, followed by storage in a ventilated or closed environment. Prior to bacterial detection from the EVA sample sheets, salivary bacterial counts were performed for five subjects, which were (3.55±0.85)×107, (2.03±0.77)×107, (4.32±1.57)×106, (9.17±1.26)×106 and (1.46±0.12)×107 CFU per 1 mL of saliva in subjects 1–5, respectively (data not shown). Time-dependent evaluation revealed no bacteria detected from samples stored 2 days or longer in a ventilated environment without cleaning (Figure 2a), as well as those washed with sterilized water (Figure 2b) and those subjected to mechanical brushing (Figure 2c). On the other hand, live bacteria were detected from samples from all five stored in a closed environment for 7 days and from samples from one of five subjects stored for 14 days (Figure 2d), while none were observed in samples following 21 and 28 days (data not shown) of storage. However, no bacteria were detected on samples stored 2 days or longer in a closed environment after washing with sterilized water (Figure 2e) or subjected to mechanical brushing (Figure 2f). The bacterial counts of the untreated samples gradually declined as the time of storage was extended. In contrast, the numbers of bacteria on samples treated with sterilized water and/or mechanical brushing rapidly decreased to none within 48 h.
Attached and surviving bacteria on surfaces of EVA sample sheets over time. Salivary bacterial counts were determined for five subjects. EVA sample sheets mixed with saliva were strongly vortexed with sterilized PBS and inoculated onto BHI agar plates, followed by storage in a ventilated (a–c) or closed (d–f) environment. Bacterial detection was performed using samples washed without any cleaning (a, d), washed with sterilized water (b, e), or washed with sterilized water following mechanical brushing (c, f). All experiments were performed in triplicate with three technical repeats. Horizontal bars indicate the standard deviation. BHI, brain–heart infusion; EVA, ethylene vinyl acetate; PBS, phosphate-buffered saline.
Bacterial identification using PCR
Bacterial identification was performed using samples obtained from two subjects (K, female; S, male) prior to storage (0 day) and after storage for 14 days. Samples were serially diluted with sterilized PBS, inoculated onto BHI agar plates, and incubated at 37 °C for 48 h. At least 100 colonies were randomly selected from each sample and identified using a PCR method. S. salivarius was mainly identified (33.0%) in the 0-day sample from S, followed by S. oralis (20.0%), S. mitis (1.0%) and others. S. oralis was dominantly detected (43.9%) in the 0-day sample from K, followed by S. salivarius (7.5%) and S. sanguinis (5.6%). After 14 days of storage in a closed environment, S. oralis was detected in more than 70% of samples from both subjects. S. salivarius was not identified in any samples after 14 days (Figure 3). In addition, S. mutans, S. sobrinus, S. pyogenes and S. pneumoniae were not identified in any of the samples under any of the experimental conditions. Primers used in this study were showed in Table 1.
Identification of bacteria isolated from EVA sample sheets stored in closed environment with or without long-term storage. Saliva samples were obtained from two subjects (K, female; S, male) at two different time points, 0 day (before storage) and after 14 days of storage in closed tubes. Next, the samples were serially diluted with PBS, inoculated onto BHI agar plates, and incubated at 37 °C for 24–48 h. At least 100 colonies were randomly selected from each sample and identified. Streptococcal species were detected using specific primer sets, as shown in Table 1. Each circle graph indicates the proportion of streptococcal species identified by PCR and undefined strains. BHI, brain–heart infusion; EVA, ethylene vinyl acetate; PBS, phosphate-buffered saline; PCR, polymerase chain reaction.
Discussion
Dental devices made of EVA are generally used repeatedly from 1 day to several months, and the method of storage is the responsibility of the user. However, no established standard for adequate storage of EVA devices has been presented. As a result, it is speculated that methods of storage differ among individuals and may not be appropriate from a bacteriological standpoint.
Bacterial contamination, survival and persistence under various harsh conditions, including desiccation, temperature, low and high pH levels, lack of nutrients, and others, have been well studied for the food processing and supply industries. The risk of bacterial cross-contamination is considered to be lower in a low-moisture environment, partly because bacterial growth and survival are reduced. However, Kusumaningrum et al.8 reported that Salmonella species survived on dried abiotic surfaces for at least 4 days. In the present study, though bacteria from salivary samples survived for 24 h, they were undetected in samples stored for 48 h or longer on the dried abiotic surfaces. To the best of our knowledge, this is the first report to present survival data for Streptococcus species, which may be helpful for other researchers.
In other previous studies, S. mitis, S. salivarius and other Streptococcus species were commonly detected in saliva obtained from different aged subjects.8,9 Interestingly, S. salivarius and S. oralis were dominantly detected in the present 0-day samples and showed a subject-dependent proportion of bacterial flora in PCR assays using the bacterial 16S rRNA gene. On the other hand, S. oralis was dominantly detected in 14-day samples, which showed a similar propensity. These results suggest that S. oralis can survive longer as compared to other oral streptococcal species.
Abiko et al.10 reported that the proportions of Streptococcus species, especially S. oralis and S. sanguinis, were higher in subgingival plaque biofilm microflora, indicating that Streptococcus species are one of the major components of subgingival plaque biofilm. S. sanguinis is considered to be the first microorganism to colonize tooth surfaces by forming dental biofilm, which is closely related to infective endocarditis caused by oral bacteria entering the bloodstream following trauma.11,12 Furthermore, S. gordonii is one of the early colonizers that initiate biofilm formation from dental plaque.13 These are reasonable explanations for the present findings, as we detected lower proportions of S. gordonii, S. mitis and S. sanguinis in salivary samples, despite previous findings that these Streptococcus species are major components of oral microflora. Furthermore, S. pyogenes is frequently obtained from throat swab specimens,14,15 and S. pneumoniae from nasopharynx and sinus carriage samples obtained from healthy and asymptomatic individuals.16,17,18 Thus, these microorganisms might be rarely observed in salivary specimens.
According to the previous study of Maeda et al.,19 oral commensal viridans group streptococci, including S. mitis, S. salivarius, S. sanguinis, S. oralis, S. gordonii and S. mutans, may potentially act as a reservoir of antibiotic resistant gene determinants for newly acquired and antibiotic-susceptible pathogens. They also frequently found several mutations that were responsible for antibiotic resistance in these organisms. Furthermore, carbohydrate restriction was suggested to provide resistance toward heat, and oxidative and acidic stresses as compared with a variety of other microorganisms.20,21,22 Thus, salivary bacteria that have been exposed to a nutrition starved condition may acquire tolerance to heat, oxidative stresses and antibiotics.
Our findings demonstrated that oral streptococci derived from saliva tend to survive in a non-ventilated moist environment. Although neither washing EVA sheets with sterilized water nor brushing was lethal for attached microbes, those methods are effective for earlier bacterial declination due weakening bacterial colonization on the EVA surface. Teixeira et al.23 previously suggested that initial colonization of tooth surfaces by streptococci involves attachment of the bacteria to adsorbed salivary components of the acquired pellicle. Cetylpyridinium chloride was effectively eradicated bacteria on the EVA surface, however, has been demonstrated to have cytotoxic side effect on several mammalian cells.24,25
Conclusion
Taken together, washing with sterilized water and a ventilated environment is effective for hygienic storage of dental devices made of EVA.
References
Maeda Y, Kumamoto D, Yagi K et al. Effectiveness and fabrication of mouthguards. Dent Traumatol 2009; 25 (6): 556–564.
Takeuchi H, Senpuku H, Matin K et al. New dental drug delivery system for removing mutans streptococci from the oral cavity: effect on oral microbial flora. Jpn J Infect Dis 2000; 53 (5): 211–212.
Craig RG, Godwin WC . Physical properties of materials for custom-made mouth protectors. J Mich State Dent Assoc 1967; 49 (2): 34–40.
Garnier F, Gerbaud G, Courvalin P et al. Identification of clinically relevant viridans group streptococci to the species level by PCR. J Clin Microbiol 1997; 35 (9): 2337–2341.
Hoshino T, Kawaguchi M, Shimizu N et al. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn Microbiol Infect Dis 2004; 48 (3): 195–199.
Ogawa T, Terao Y, Sakata H et al. Epidemiological characterization of Streptococcus pyogenes isolated from patients with multiple onsets of pharyngitis. FEMS Microbiol Lett 2011; 318 (2): 143–151.
Prère MF, Fayet OA . A specific polymerase chain reaction test for the identification of Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2011; 70 (1): 45–53.
Kusumaningrum HD, Riboldi G, Hazeleger WC et al. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol 2003; 85 (3): 227–236.
Kang JG, Kim SH, Ahn TY . Bacterial diversity in the human saliva from different ages. J Microbiol 2006; 44 (5): 572–576.
Abiko Y, Sato T, Mayanagi G et al. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res 2010; 45 (3): 389–395.
Douglas CW, Heath J, Hampton KK et al. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol 1993; 39 (3): 179–182.
Yamaguchi M, Terao Y, Ogawa T et al. Role of Streptococcus sanguinis sortase A in bacterial colonization. Microbes Infect 2006; 8 (12/13): 2791–2796.
Kuboniwa M, Tribble GD, James CE et al. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol 2006; 60 (1): 121–139.
Begovac J, Bobinac E, Benic B et al. Asymptomatic pharyngeal carriage of beta-haemolytic streptococci and streptococcal pharyngitis among patients at an urban hospital in Croatia. Eur J Epidemiol 1993; 9 (4): 405–410.
Cockerill FR 3rd, Macdonald KL, Thompson RL et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 1997; 277 (1): 38–43.
Quintero B, Araque M, van der Gaast–de Jongh C et al. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur J Clin Microbiol Infect Dis 2011; 30 (1): 7–19.
Farrell DJ, Klugman KP, Pichichero M . Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J 2007; 26 (2): 123–128.
Yamaguchi M, Minamide Y, Terao Y et al. Nrc of Streptococcus pneumoniae suppresses capsule expression and enhances anti-phagocytosis. Biochem Biophys Res Commun 2009; 390 (1): 155–160.
Maeda Y, Murayama M, Goldsmith CE et al. Molecular characterization and phylogenetic analysis of quinolone resistance-determining regions (QRDRs) of gyrA, gyrB, parC and parE gene loci in viridans group streptococci isolated from adult patients with cystic fibrosis. J Antimicrob Chemother 2011; 66 (3): 476–486.
Giard JC, Hartke A, Flahaut S et al. Starvation-induced multiresistance in Enterococcus faecalis JH2–2. Curr Microbiol 1996; 32 (5): 264–271.
Givskov M, Eberl L, Møller S et al. Responses to nutrient starvation in Pseudomonas putida KT2442: analysis of general cross-protection, cell shape, and macromolecular content. J Bacteriol 1994; 176 (1): 7–14.
Hartke A, Bouche S, Gansel X et al. Starvation-induced stress resistance in Lactococcus lactis subsp. lactis IL1403. Appl Environ Microbiol 1994; 60 (9): 3474–3478.
Teixeira EH, Napimoga MH, Carneiro VA et al. In vitro inhibition of Streptococci binding to enamel acquired pellicle by plant lectins. J Appl Microbiol 2006; 101 (1): 111–116.
Müller G, Kramer A . Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 2008; 61 (6): 1281–1287.
Kano S, Sugibayashi K . Kinetic analysis on the skin disposition of cytotoxicity as an index of skin irritation produced by cetylpyridinium chloride: comparison of in vitro data using a three-dimensional cultured human skin model with in vivo results in hairless mice. Pharm Res 2006; 23 (2): 329–335.
Acknowledgements
We thank Drs T Fujio, M Kankubo, Y Miyashita, T Suganami and M Sugie for providing the salivary samples. This study was supported in part by grants from Altimate Co. Ltd, Kensinkai Healthcare Corp., Platon Japan Co. Ltd, Straumann Japan K. K., T. U. M. Co. Ltd and Yamahachi Dental Mfg. Co.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ogawa, T., Yamasaki, S., Honda, M. et al. Long-term survival of salivary streptococci on dental devices made of ethylene vinyl acetate. Int J Oral Sci 4, 14–18 (2012). https://doi.org/10.1038/ijos.2012.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2012.13
Keywords
This article is cited by
-
Microbial Contamination and Disinfection of Sport Mouthguard: In Vitro Study
Current Microbiology (2020)
-
Influence of sport mouthguards on the ecological factors of the children oral cavity
BMC Oral Health (2014)