Abstract
Background/Objectives:
Mutations in the Tubby gene (TUB) cause late-onset obesity and insulin resistance in mice and syndromic obesity in humans. Although TUB gene function has not yet been fully elucidated, studies in rodents indicate that TUB is involved in the hypothalamic pathways regulating food intake and adiposity. Aside from the function in central nervous system, TUB has also been implicated in energy metabolism in adipose tissue in rodents. We aimed to determine the expression and distribution patterns of TUB in man as well as its potential association with obesity.
Subjects/Methods:
In situ hybridization was used to localize the hypothalamic regions and cells expressing TUB mRNA. Using RT–PCR, we determined the mRNA expression level of the two TUB gene alternative splicing isoforms, the short and the long transcript variants, in the hypothalami of 12 obese and 12 normal-weight subjects, and in biopsies from visceral (VAT) and subcutaneous (SAT) adipose tissues from 53 severely obese and 24 non-obese control subjects, and correlated TUB expression with parameters of obesity and metabolic health.
Results:
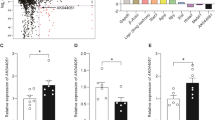
Expression of both TUB transcripts was detected in the hypothalamus, whereas only the short TUB isoform was found in both VAT and SAT. TUB mRNA was detected in several hypothalamic regions involved in body weight regulation, including the nucleus basalis of Meynert and the paraventricular, supraoptic and tuberomammillary nuclei. We found no difference in the hypothalamic TUB expression between obese and control groups, whereas the level of TUB mRNA was significantly lower in adipose tissue of obese subjects as compared to controls. Also, TUB expression was negatively correlated with indices of body weight and obesity in a fat-depot-specific manner.
Conclusions:
Our results indicate high expression of TUB in the hypothalamus, especially in areas involved in body weight regulation, and the correlation between TUB expression in adipose tissue and obesity. These findings suggest a role for TUB in human obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boggon TJ, Shan WS, Santagata S, Myers SC, Shapiro L . Implication of tubby proteins as transcription factors by structure-based functional analysis. Science 1999; 286: 2119–2125.
Coleman DL, Eicher EM . Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered 1990; 81: 424–427.
Kleyn PW, Fan W, Kovats SG, Lee JJ, Pulido JC, Wu Y et al. Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell 1996; 85: 281–290.
Noben-Trauth K, Naggert JK, North MA, Nishina PM . A candidate gene for the mouse mutation tubby. Nature 1996; 380: 534–538.
Stubdal H, Lynch CA, Moriarty A, Fang Q, Chickering T, Deeds JD et al. Targeted deletion of the tub mouse obesity gene reveals that tubby is a loss-of-function mutation. Mol Cell Biol 2000; 20: 878–882.
Kapeller R, Moriarty A, Strauss A, Stubdal H, Theriault K, Siebert E et al. Tyrosine phosphorylation of tub and its association with Src homology 2 domain-containing proteins implicate tub in intracellular signaling by insulin. J Biol Chem 1999; 274: 24980–24986.
Prada PO, Quaresma PG, Caricilli AM, Santos AC, Guadagnini D, Morari J et al. Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei. Diabetes 2013; 62: 137–148.
Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS et al. G-protein signaling through tubby proteins. Science 2001; 292: 2041–2050.
Ikeda A, Nishina PM, Naggert JK . The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci 2002; 115: 9–14.
Sahly I, Gogat K, Kobetz A, Marchant D, Menasche M, Castel M et al. Prominent neuronal-specific tub gene expression in cellular targets of tubby mice mutation. Hum Mol Genet 1998; 7: 1437–1447.
Berthoud HR . Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 2002; 26: 393–428.
Backberg M, Madjid N, Ogren SO, Meister B . Down-regulated expression of agouti-related protein (AGRP) mRNA in the hypothalamic arcuate nucleus of hyperphagic and obese tub/tub mice. Brain Res Mol Brain Res 2004; 125: 129–139.
Guan XM, Yu H, Van der Ploeg LH . Evidence of altered hypothalamic pro-opiomelanocortin/ neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res 1998; 59: 273–279.
Backberg M, Meister B . Abnormal cholinergic and GABAergic vascular innervation in the hypothalamic arcuate nucleus of obese tub/tub mice. Synapse 2004; 52: 245–257.
Park J, Lee J, Shim J, Han W, Bae YC, Chung YD et al. dTULP, the Drosophila melanogaster homolog of tubby, regulates transient receptor potential channel localization in cilia. PLoS Genet 2013; 9: e1003814.
Sun X, Haley J, Bulgakov OV, Cai X, McGinnis J, Li T . Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia 2012; 1: 21.
Coyle CA, Strand SC, Good DJ . Reduced activity without hyperphagia contributes to obesity in Tubby mutant mice. Physiol Behav 2008; 95: 168–175.
Wang Y, Seburn K, Bechtel L, Lee BY, Szatkiewicz JP, Nishina PM et al. Defective carbohydrate metabolism in mice homozygous for the tubby mutation. Physiol Genomics 2006.
Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA . C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab 2005; 2: 35–42.
Stretton C, Litherland GJ, Moynihan A, Hajduch E, Hundal HS . Expression and modulation of TUB by insulin and thyroid hormone in primary rat and murine 3T3-L1 adipocytes. Biochem Biophys Res Commun 2009; 390: 1328–1333.
Shiri-Sverdlov R, Custers A, van Vliet-Ostaptchouk JV, van Gorp PJ, Lindsey PJ, van Tilburg JH et al. Identification of TUB as a novel candidate gene influencing body weight in humans. Diabetes 2006; 55: 385–389.
van Vliet-Ostaptchouk JV, Onland-Moret NC, Shiri-Sverdlov R, van Gorp PJ, Custers A, Peeters PH et al. Polymorphisms of the TUB gene are associated with body composition and eating behavior in middle-aged women. PLoS ONE 2008; 3: e1405.
Borman AD, Pearce LR, Mackay DS, Nagel-Wolfrum K, Davidson AE, Henderson R et al. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat 2014; 35: 289–293.
Braak H, Braak E . Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259.
Vester B, Wengel J . LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry 2004; 43: 13233–13241.
Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z . RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA 2006; 12: 187–191.
Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A . Melanocortin 4 receptor distribution in the human hypothalamus. Eur J Endocrinol 2013; 168: 361–369.
Durrenberger PF, Fernando FS, Magliozzi R, Kashefi SN, Bonnert TP, Ferrer I et al. Selection of novel reference genes for use in the human central nervous system: a BrainNet Europe Study. Acta Neuropathol 2012; 124: 893–903.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Szalowska E, Elferink MG, Hoek A, Groothuis GM, Vonk RJ . Resistin is more abundant in liver than adipose tissue and is not up-regulated by lipopolysaccharide. J Clin Endocrinol Metab 2009; 94: 3051–3057.
Wolfs MG, Rensen SS, Bruin-Van Dijk EJ, Verdam FJ, Greve JW, Sanjabi B et al. Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study. BMC Med Genomics 2010; 3: 34.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: research0034.1–research0034.11.
Swaab DF. 2003The human hypothalamus: basic and clinical aspects - Part 1: nuclei of the human hypothalamus. In: Aminoff MJ, Boller F, Swaab DF ( eds). Handbook of Clinical Neurology, vol. 79. Elsevier: Amsterdam, The Netherlands, 2003, pp 1–479.
Alkemade A, Yi CX, Pei L, Harakalova M, Swaab DF, la Fleur SE et al. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in alphaMSH are related to type 2 diabetes. J Clin Endocrinol Metab 2012; 97: E925–E933.
Oh EC, Vasanth S, Katsanis N . Metabolic regulation and energy homeostasis through the primary Cilium. Cell Metab 2015; 21: 21–31.
Scherer PE . Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006; 55: 1537–1545.
Kloting N, Schleinitz D, Ruschke K, Berndt J, Fasshauer M, Tonjes A et al. Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia 2008; 51: 641–647.
Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006; 14: 16S–19S.
Snieder H, Wang X, Shiri-Sverdlov R, van Vliet-Ostaptchouk JV, Hofker MH, Perks U et al. TUB is a candidate gene for late-onset obesity in women. Diabetologia 2008; 51: 54–61.
Koritschoner NP, Alvarez-Dolado M, Kurz SM, Heikenwalder MF, Hacker C, Vogel F et al. Thyroid hormone regulates the obesity gene tub. EMBO Rep 2001; 2: 499–504.
Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 2006; 3: 135–140.
Yang K, Guan H, Arany E, Hill DJ, Cao X . Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J 2008; 22: 2452–2464.
Pellegrinelli V, Carobbio S, Vidal-Puig A . Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 2016; 59: 1075–1088.
Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One 2011; 6: e17154.
Acknowledgements
We thank our colleagues Elinda J Bruin-Van Dijk for expression profiling of adipose tissues, Bahram Sanjabi for determining the RQS values and Vincent W Bloks for critical comments on the manuscript and statistical advice. We thank Sally Hill (Scientific Texts) for critical reading and editing of the manuscript. JV van Vliet-Ostaptchouk is supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (project no. 2013.81.1673). This work was supported by the National Consortium for Healthy Ageing (NCHA) (NCHA NGI Grant 050-060-810), and the European Union’s Seventh Framework programme (FP7/2007–2013) through the BioSHaRE-EU (Biobank Standardisation and Harmonisation for Research Excellence in the European Union) project, grant agreement 261433 and by grants from the Dutch Diabetes Foundation (grant 2012.00.1537 to JWJ) and The Netherlands Organization for Scientific Research (VIDI grant 016.126.338 to JWJ).
Author contributions
JVvVO conceived and designed the experiments. VJMN, JvVO wrote the paper. VJMN, DS, MGMW, JVvVO performed the experiments. JVvVO, VJMN, DS, MGMW, TPvdM, UU, DFS analyzed the data. DFS, BHRW, JWJ critically revised the article for important intellectual content. SSR, ES, DFS, BHRW, JWJ contributed to the interpretation of the data. SSR, ES, UU, KF, GH, WAB, JWG, FR, RSS, RJV, DFS, BHRW, JWJ contributed study materials/reagents/materials. All authors read and approved the final manuscript. VJMN and JVvVO are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Nies, V., Struik, D., Wolfs, M. et al. TUB gene expression in hypothalamus and adipose tissue and its association with obesity in humans. Int J Obes 42, 376–383 (2018). https://doi.org/10.1038/ijo.2017.214
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.214