Abstract
Background:
The notion that hepatic expression of genes involved in lipid metabolism is altered in obese patients is relatively new and its relationship with hepatic steatosis and cardiometabolic alterations remains unclear.
Objective:
We assessed the impact of Roux-en-Y gastric bypass surgery (RYGB) on the expression profile of genes related to metabolic syndrome in liver biopsies from morbidly obese individuals using a custom-made, focused cDNA microarray, and assessed the relationship between the expression profile and hepatic steatosis regression.
Materials and methods:
Plasma and liver samples were obtained from patients at baseline and 12 months after surgery. Samples were assayed for chemical and gene expression analyses, as appropriate. Gene expression profiles were assessed using custom-made, focused TaqMan low-density array cards.
Results:
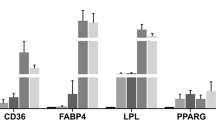
RYGB-induced weight loss produced a favorable reduction in fat deposits, insulin resistance (estimated by homeostasis model assessment of insulin resistance (HOMA-IR)), and plasma and hepatic lipid levels. Compared with the baseline values, the gene expression levels of key targets of lipid metabolism were significantly altered: CD36 was significantly downregulated (−40%; P=0.001), whereas APOB (+27%; P=0.032) and SCARB1 (+37%; P=0.040) were upregulated in response to surgery-induced weight reduction. We also observed a favorable reduction in the expression of the PAI1 gene (−80%; P=0.007) and a significant increase in the expression of the PPARA (+60%; P=0.014) and PPARGC1 genes (+36%; P=0.015). Notably, the relative fold decrease in the expression of the CD36 gene was directly associated with a concomitant reduction in the cholesterol (Spearman’s r=0.92; P=0.001) and phospholipid (Spearman’s r=0.76; P=0.04) contents in this tissue.
Conclusions:
For the first time, RYGB-induced weight loss was shown to promote a favorable downregulation of CD36 expression, which was proportional to a favorable reduction in the hepatic cholesterol and phospholipid contents in our morbidly obese subjects following surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112: 2735–2752.
Vander Naalt SJ, Gurria JP, Holterman AL . Surgical treatment of nonalcoholic fatty liver disease in severely obese patients. Hepat Med 2014; 6: 103–112.
Knop FK, Taylor R . Mechanism of metabolic advantages after bariatric surgery: it's all gastrointestinal factors versus it's all food restriction. Diabetes Care 2013; 36: S287–S291.
Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009; 137: 532–540.
Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC . Effect of bariatric surgery on liver fibrosis. Obes Surg 2012; 22: 1044–1049.
Nostedt JJ, Switzer NJ, Gill RS, Dang J, Birch DW, de Gara C et al. The effect of bariatric surgery on the spectrum of fatty liver disease. Can J Gastroenterol Hepatol 2016; 2016: 2059245.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023.
Vargas V, Allende H, Lecube A, Salcedo MT, Baena-Fustegueras JA, Fort JM et al. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J Hepatol 2012; 4: 382–388.
Alessi MC, Bastelica D, Mavri A, Morange P, Berthet B, Grino M et al. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol 2003; 23: 1262–1268.
Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut 2010; 59: 1259–1264.
Ferrer R, Pardina E, Rossell J, Baena-Fustegueras JA, Lecube A, Balibrea JM et al. Decreased lipases and fatty acid and glycerol transporter could explain reduced fat in diabetic morbidly obese. Obesity 2014; 22: 2379–2387.
Ferrer R, Pardina E, Rossell J, Baena-Fustegueras JA, Lecube A, Balibrea JM et al. Haematological parameters and serum trace elements in ‘healthy’ and ‘unhealthy’ morbidly obese patients before and after gastric bypass. Clin Nutr 2014; 34: 276–283.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001; 285: 2486–2497.
Lohman T, Roche A, Martorel R Standardization of Anthropometric Measurements. Human Kinetics Publishers: Champaign, IL, 1988.
Bonora E, Micciolo R, Ghiatas AA, Lancaster JL, Alyassin A, Muggeo M et al. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism 1995; 44: 1617–1625.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Pardina E, Ferrer R, Baena-Fustegueras JA, Lecube A, Fort JM, Vargas V et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg 2010; 20: 623–632.
Hara A, Radin NS . Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90: 420–426.
Hafeez S, Ahmed MH . Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes 2013; 2013: 839275.
Lutz TA, Bueter M . The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol 2014; 307: R1275–R1291.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report Of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002; 106: 3143–3421.
Pardina E, Baena-Fustegueras JA, Llamas R, Catalan R, Galard R, Lecube A et al. Lipoprotein lipase expression in livers of morbidly obese patients could be responsible for liver steatosis. Obes Surg 2009; 19: 608–616.
Vernon G, Baranova A, Younossi ZM . Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285.
Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol 2008; 294: G1281–G1287.
Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011; 60: 1394–1402.
Memon RA, Fuller J, Moser AH, Smith PJ, Grunfeld C, Feingold KR . Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes 1999; 48: 121–127.
Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007; 56: 2863–2871.
Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 2008; 134: 556–567.
Sheedfar F, Sung MM, Aparicio-Vergara M, Kloosterhuis NJ, Miquilena-Colina ME, Vargas-Castrillon J et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging 2014; 6: 281–295.
Jay AG, Hamilton JA . The enigmatic membrane fatty acid transporter CD36: New insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot Essent Fatty Acids 2016; e-pub ahead of print 20 May 2016 doi:10.1016/j.plefa.2016.05.005.
Musso G, Gambino R, Cassader M . Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res 2009; 48: 1–26.
Love-Gregory L, Abumrad NA . CD36 genetics and the metabolic complications of obesity. Curr Opin Clin Nutr Metab Care 2011; 14: 527–534.
Pettinelli P, Videla LA . upregulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab 2011; 96: 1424–1430.
Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Soderstrom I, Edlund H . Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem 2015; 290: 19034–19043.
Souza-Mello V . Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J Hepatol 2015; 7: 1012–1019.
Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 2005; 336: 215–222.
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD . Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113: 1582–1588.
Tontonoz P, Spiegelman BM . Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008; 77: 289–312.
Gastaldi G, Russell A, Golay A, Giacobino JP, Habicht F, Barthassat V et al. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia 2007; 50: 2348–2355.
Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 2004; 110: 3259–3269.
Rao MS, Reddy JK . PPARalpha in the pathogenesis of fatty liver disease. Hepatology 2004; 40: 783–786.
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005; 3: e101.
Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016; 351: 1166–1171.
Rosenson RS, Brewer Jr HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 2016; 13: 48–60.
Vaneckova I, Maletinska L, Behuliak M, Nagelova V, Zicha J, Kunes J . Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol 2014; 223: R63–R78.
Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol 2014; 61: 148–154.
Targher G, Zoppini G, Moghetti P, Day CP . Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Semin Thromb Hemost 2010; 36: 41–48.
Cigolini M, Targher G, Agostino G, Tonoli M, Muggeo M, De Sandre G . Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men—role of the metabolic syndrome. Thromb Haemost 1996; 76: 69–73.
Pardina E, Ferrer R, Rivero J, Baena-Fustegueras JA, Lecube A, Fort JM et al. Alterations in the common pathway of coagulation during weight loss induced by gastric bypass in severely obese patients. Obesity 2012; 20: 1048–1056.
Makki K, Froguel P, Wolowczuk I . Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013; 2013: 139239.
Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014; 59: 121–129.
Singh P, Peterson TE, Barber KR, Kuniyoshi FS, Jensen A, Hoffmann M et al. Leptin upregulates the expression of plasminogen activator inhibitor-1 in human vascular endothelial cells. Biochem Biophys Res Commun 2010; 392: 47–52.
Devaraj S, Xu DY, Jialal I . C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation 2003; 107: 398–404.
Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G . High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol 2010; 16: 6119–6122.
Stine JG, Argo CK, Pelletier SJ, Maluf DG, Caldwell SH, Northup PG . Advanced non-alcoholic steatohepatitis cirrhosis: a high-risk population for pre-liver transplant portal vein thrombosis. World J Hepatol 2017; 9: 139–146.
Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG . Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl 2015; 21: 1016–1021.
Acknowledgements
This work was funded by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (ISCIII) FIS grants CP13/00070 (to JJ) and PI11/01159 and PI15/00190 (to JP-O), and FEDER ‘Una manera de hacer Europa’; and by LaMarató 2016 (303/C/2016) (to JJ). JJ is recipient of a Miguel Servet Type 1 contract (CP13/00070; ISCIII). KAM-L is recipient of a AGAUR grant FI-DGR2014 (Generalitat de Catalunya). CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) is a project of the Instituto de Salud Carlos III. Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau is accredited by the Generalitat de Catalunya as Centre de Recerca de Catalunya (CERCA). The English grammar and language was corrected by American Journal Experts (www.aje.com).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Pardina, E., Ferrer, R., Rossell, J. et al. Hepatic CD36 downregulation parallels steatosis improvement in morbidly obese undergoing bariatric surgery. Int J Obes 41, 1388–1393 (2017). https://doi.org/10.1038/ijo.2017.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.115
This article is cited by
-
Pathogenic effects of Desulfovibrio in the gut on fatty liver in diet-induced obese mice and children with obesity
Journal of Gastroenterology (2022)
-
Administration of N-Acyl-Phosphatidylethanolamine Expressing Bacteria to Low Density Lipoprotein Receptor−/− Mice Improves Indices of Cardiometabolic Disease
Scientific Reports (2019)