Abstract
Background/Objectives:
A number of recent studies dealing with the relationship between the effects of high body mass (BM) and fat mass (FM) on bone mass and strength exhibit a range of contrasting variations in their findings. These diverse findings have led to an ongoing controversy as to whether high BM and FM positively or negatively affect bone mass and strength. Excessive FM and the associated low-grade inflammation might overturn the higher mechanical stimulus arising from a higher BM. Therefore, we aimed at quantifying the functional muscle–bone unit in premenopausal women with markedly diverging body composition.
Subjects/Methods:
Sixty-four young women with BMs ranging from 50 to 113 kg and body fat percentages between 20.7% and 51.8% underwent jumping mechanography and peripheral quantitative computed tomography measurements. Maximum voluntary ground reaction force during multiple one-legged hopping (Fm1LH), as well as bone characteristics at 4, 14 and 38% of tibia length, were determined. Body composition was assessed by dual-energy X-ray absorptiometry, and serum inflammatory markers were analyzed from blood samples.
Results:
Fm1LH predicted volumetric bone mineral content at the 14% site by 48.7%. Women with high body fat percentage had significantly higher Fm1LH, significantly lower relative bone mass, relative bone strength and relative bone area, as well as higher serum inflammatory markers in comparison to women with lower body fat percentage.
Conclusions:
In conclusion, high body fat percentage was associated with lower relative bone mass and strength despite normal habitual muscle force in premenopausal women, indicating that high body fat percentage compromised the functional muscle–bone unit in these individuals.
Similar content being viewed by others
Introduction
According to the mechanostat theory, mechanical usage affects bone mass and geometry by way of a negative feedback system.1 The proposed effect of bone homeostasis may represent an adaptive process to keep bone strain close to a set point.2 Although it remains to be demonstrated empirically that muscle forces represent the primary stimulus driving bone adaptation, convincing evidence posits that maximal habitual muscle forces are capable of accounting for most of the bone’s adaptive responses.3, 4, 5 These research findings indicate that a strong correlation between bone mass and geometry and maximal habitual muscle force exists and posit that muscle and bone build a functional unit. This idea has been corroborated in a series of previous studies in which jumping mechanography was used in conjunction with peripheral quantitative computed tomography (pQCT) to estimate maximal habitual force and bone strength, respectively. For instance, we showed in a large cross-sectional study comprising 323 male and female participants between 8 and 88 years of age that maximum voluntary ground reaction force during multiple one-legged hopping (Fm1LH) predicted volumetric bone mineral content (vBMC) at 14% of tibia length by 84.0%.6 This finding was confirmed in schoolchildren,7 adolescent soccer players,8 formerly anorexic women in long-term remission9 and in female children and adolescents with Turner syndrome.10
Taken together, these results indicate that the functional muscle–bone unit is independent of gender and age and that it proves true throughout a large range of loading conditions. However, within the feedback system, several mechanical and non-mechanical modulators (for example, body composition, systemic inflammation) might influence the mechanostat’s effects on bone strength3 and might affect the relationship between bone and muscle force. In particular, it is postulated that an increased body mass (BM) leads to a higher mechanical loading, which in turn causes a higher areal bone mineral density11, 12 and a higher total body BMC.13 However, people with a higher body fat percentage exhibit a lower total body BMC.13 Collectively, these results suggest that, overall, increased BM is associated with increased bone mass but that, at the same time, increases in fat mass (FM) may blunt this effect. Several insights into the biological functions of adipose tissue may lend further credence to the notion that a higher body fat percentage can negatively affect bone mass. On the one hand, adipocytes and osteoblasts are derived from the same mesenchymal stem cells.14 Based on the observation that mesenchymal stem cells might be directed to undergo adipogenic instead of osteogenic differentiation, a distinctive increase in adipocytes might impair osteoblastic differentiation.15 On the other hand, adipose tissue acts as an endocrine organ,16 whereby the upregulation of several pro-inflammatory cytokines increases bone resorption and might lead to a reduced areal bone mineral density.17
The knowledge of the status of the functional muscle–bone unit is an important prerequisite for implementing adequate interventions to restore muscle–bone function.18 Based on existing data, the effect of an excessive increase in FM on the functional muscle–bone unit is largely unknown. On the backdrop of the existing data, our study aimed at investigating whether the bone of premenopausal women with widely differing body composition is adapted to the acting maximum muscle force. Based on the underlying mechanisms of bone adaptation, we hypothesized that bone mass and bone strength are negatively affected by a high body fat percentage and/or elevated values for serum inflammatory markers.
Methods
Participants
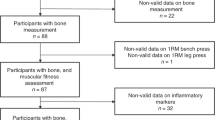
Sixty-four women were recruited for this study by placard. Eligibility criteria were female gender, aged between 18 and 45 years, non-smoker, non-pregnant and no known metabolic or other chronic diseases. Recruited participants were (mean±s.d.) aged 26.8±5.4 years, had a BM of 75.7±14.5 kg and were 167.3±6.5 cm tall. After completing a routine health questionnaire, the participants were informed about the applied procedures and about the associated risks. The participants provided written informed consent for participation in this study. All experiments were approved by the local ethics committee and the study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki for human experimentation. To analyze the effect of body fat percentage on distinct bone variables, the participants were retrospectively assigned to one of the three body fat percentage groups (Table 1): <30% body fat (n=19, age: 24.5±3.9 years, height: 166±5 cm), 30–40% body fat (n=21, age: 25.5±3.3 years, height: 169±6 cm), and >40% body fat (n=24, 29.8±6.6 years, 167±8 cm). As a reference for the healthy, non-deconditioned status of the functional muscle–bone unit, we included reference data from previously studied cohorts.6, 7, 18
Peripheral quantitative computed tomography
An XCT 3000 Scanner (Stratec, Pforzheim, Germany) was used for pQCT measurements. Section images were obtained from the calf scout view of the right tibio-talar joint. Scans were obtained at 4% (epiphysis), 14% and 38% (diaphysis) of tibia length. The length of the tibia of the non-dominant leg was measured based on anatomical landmarks (from knee joint line to medial malleolus) using a ruler. For all pQCT measurements, the angle between the foot and tibia was adjusted to 120°. Images were analyzed with the integrated XCT software in its version 6.00. vBMC was assessed with the detection threshold set to 180 and 710 mg cm−3 for epiphyseal and diaphyseal scans, respectively.
Jumping mechanography
The participants were instructed to remove their shoes, stand with feet shoulder width apart and arms hanging loosely at their sides. Fm1LH was determined by multiple one-legged hopping on the non-dominant leg as previously described6, 18 on a strain gauge ground reaction force platform (Leonardo Mechanograph, Novotec, Pforzheim, Germany) linked to a desktop computer using an integrated analog digital board and software system (Leonardo Mechanography GRFP version 4.2, Novotec, Pforzheim, Germany). Briefly, approximately 15 repeated jumps on the forefoot with a stiff knee were performed. Any jumps with heel contact were excluded from the analysis. Heel contact was controlled visually during the jumping maneuver and was additionally detected by the software. Fm1LH corresponded to maximum voluntary ground reaction force during multiple one-legged hopping.
Dual-energy X-ray absorptiometry
A densitometer (Lunar iDXA, GE Healthcare, Madison, WI, USA) was used for the determination of body composition according to the manufacturer’s specifications. Scan analysis was performed using the GE encore software version 11.40.004. Body fat percentage was determined from tissue mass (FM+lean mass).
Blood analysis
Venous blood samples were drawn into EDTA-coated and trace element-free Vacutainer tubes. Enzyme-linked immunosorbent assay technique19 was used to assess high-sensitive C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP). Interleukin-6 (IL-6) was measured by a Quantikine ELISA Kit (R&D systems, Minneapolis, MN, USA).
Statistical analysis
Data are presented as mean±s.d. Normality of data was visually analyzed by Q-Q-Plots. To detect differences between fat percentage groups, a one-way analysis of variance with Bonferroni correction was applied. Pearson correlations were performed to test for associations between different variables and the adjusted R2 is provided. Analysis of covariance was used to detect significant differences between slopes and intercepts of regression lines. For statistical analysis, SPSS 23.0 statistical software (SPSS, Chicago, IL, USA) was used. A P<0.05 was set as statistical significance.
Results
Overall, Fm1LH predicted the variability in vBMC at the 14% site by 48.7% (Figure 1a). The slope of the regression line between vBMC at the 14% site and Fm1LH was significantly different from the slope of the regression line of the reference population, consisting of young and healthy children and adolescents (F(1, 207)=23.7, P<0.001). There was a significant negative correlation between Frelm1LH and BM (Figure 1b) as well as between Frelm1LH and FM (y=−0.0272x+3.751, R2=0.536, P<0.001). There was a significant difference in Fm1LH between women with >40% body fat compared with women with <30% body fat (Table 1). Frelm1LH was significantly higher in women with <30% body fat as compared with women with 30–40% body fat and women with >40% body fat. In addition, Frelm1LH was significantly higher in women with 30–40% body fat as compared with women with >40% body fat.
(a) Relationship between volumetric bone mineral content at 14% of tibia length (vBMC14%) and maximum voluntary ground reaction force (Fm1LH) in 64 women. The dashed line represents the mean value and the dotted lines the 95% prediction bands for healthy children and adolescents.18 (b) Relationship between maximum voluntary ground reaction force normalized to body weight (Frelm1LH) and BM in 64 women. The dashed line and dotted lines shows the mean value and s.d. for healthy children and adolescents, respectively.18
CRP and AGP values were significantly higher in women with >40% body fat as compared with women with <30% body fat. AGP and IL-6 values were significantly higher in women with >40% body fat than in women with 30–40% body fat. There were significant correlations between fat percentage and AGP values (y=0.153x+0.227, adj. R2=0.191, P<0.001), CRP values (y=0.008x+0.033, adj. R2=0.213, P<0.001) and IL-6 values (y=0.031x+0.332, adj. R2=0.049, P=0.044), respectively. We also found significantly negative correlations between all relative bone variables and both CRP and AGP, but not IL-6, plasma concentrations (data not shown). For instance, relative vBMC at the 14% site correlated with CRP (y=−0.0006x+0.0365, adj. R2=0.194, P<0.001) and AGP (y=−0.0110x+0.0441, adj. R2=0.204, P<0.001) plasma concentration.
At all measured positions, relative vBMC, relative volumetric bone mineral density, relative bone area and relative strain-strength-index were lower in women with 30–40% body fat as compared with women with <30% body fat (Table 1). Women with >40% body fat had significantly lower values for all the assessed relative bone variables as compared with women with <30% body fat as well as compared with women with 30–40% body fat (Table 1).
Discussion
Several new findings could be obtained through this cross-sectional study in premenopausal women. First, Fm1LH predicted vBMC at the 14% site by 48.7% in women with markedly divergent body compositions. Second, bone variables adjusted for BM were significantly lower in women with >30% body fat as compared with women with <30% body fat. Third, negative correlations between all relative bone variables and CRP as well as AGP were present.
Overall, Fm1LH predicted the variability in vBMC at 14% of tibia length by almost 50%. This correlation between Fm1LH and vBMC at 14% of tibia length was present, albeit the BMs of the participants ranged from 50 to 113 kg, and body fat percentages were between 20% and 52%. More importantly, the data points for most women were located within the 95% prediction bands of young and healthy children and adolescents. Therefore, bone mass was generally adapted to the acting muscle forces in most of our participants. However, we found that women with a higher body fat percentage had significantly lower values for all bone variables adjusted for BM. This finding challenges the intact muscle–bone relationship in women with high body fat percentage, because their Fm1LH was significantly higher than their counterparts with lower body fat percentage. It might be argued that relative bone variables were already lower in women with high body fat percentage at the end of puberty. However, the significant correlations between Frelm1LH and BM as well as FM disprove this argument. Moreover, a higher Fm1LH in conjunction with lower bone mass and strength would contradict the theoretical mechanisms of bone accretion during childhood and adolescence,2, 20, 21 which posit that during childhood and adolescence, joint size adapts to maximum voluntary muscle force and gravitational force.22, 23, 24 After closure of growth plates, joint size and hyaline cartilage material properties cannot, under normal conditions, be further increased.21, 25, 26 Therefore, the higher Fm1LH in women with higher body fat percentage as compared with women with lower body fat percentage might be indicative of a larger joint size and, consequently, higher absolute bone mass and strength would be expected.
In women with a high body fat percentage, two modulators might be taken into account for the lower bone mass and bone strength. First, an extensive increase in FM might per se lead to a decrease in bone mass. A postadolescent, excessive increase in FM might direct a large part of the mesenchymal stem cells into adipocytes and only a smaller amount into osteoblasts. In contrast to osteoblasts, osteoclasts have origin in the self-fusion of macrophages27 and are not dependent upon alterations in fat tissue. Consequently, a disproportional increase in FM might result in a net degradation of bone mass. The negative correlation between Frelm1LH and FM in this study indicated that a large part of the FM increase occurred after the end of puberty. Moreover, the significant negative correlation between Frelm1LH and BM was only present in women with >30% body fat. Second, chronic inflammation might account for a raise in bone resorptive mechanisms. In this regard, we found significant correlations between body fat percentage and all measured serum inflammatory markers. In addition, women with >40% body fat had higher values for all serum inflammatory markers, as compared with women with lower body fat percentage. Our result that CRP plasma concentration was negatively correlated with relative bone mass and strength values is in contrast to a previous report28 but is supported by the outcomes of a multitude of studies.17, 29, 30, 31 Hence, negative correlations between CRP and relative bone variables, as found in this study, may represent an early indicator for the negative effect of chronic inflammation, which after prolonged exposure might result in decreased absolute bone mass and strength, as reported in other studies.17, 29, 30, 31 In summary, we conclude that high body fat percentage was associated with lower relative bone mass and strength despite normal habitual muscle force in premenopausal women, indicating that high body fat percentage compromised the functional muscle–bone unit in these individuals.
References
Frost HM . The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 1987; 2: 73–85.
Frost HM . Bone ‘mass’ and the ‘mechanostat’: a proposal. Anat Rec 1987; 219: 1–9.
Frost HM . Bone’s mechanostat: a 2003 update. Anat Rec A 2003; 275: 1081–1101.
Robling AG . Is bone’s response to mechanical signals dominated by muscles forces? Med Sci Sports Exerc 2009; 41: 2044–2049.
Schiessl H, Frost HM, Jee WS . Estrogen and bone-muscle strength and mass relationships. Bone 1998; 22: 1–6.
Anliker E, Rawer R, Boutellier U, Toigo M . Maximum ground reaction forces in relation to tibial bone mass in children and adults. Med Sci Sports Exerc 2011; 43: 2102–2109.
Anliker E, Dick C, Rawer R, Toigo M . Effects of jumping exercise on maximum ground reaction force and bone in 8- to 12-year-old boys and girls: a 9-month randomized controlled trial. J Musculoskelet Neuronal Interact 2012; 12: 56–67.
Anliker E, Sonderegger A, Toigo M . Side-to-side differences in the lower leg muscle-bone unit in male soccer players. Med Sci Sports Exerc 2013; 45: 1545–1552.
Mueller SM, Immoos M, Anliker E, Drobnjak S, Boutellier U, Toigo M . Reduced bone strength and muscle force in women 27 years after anorexia nervosa. J Clin Endocrinol Metab 2015; 100: 2927–2933.
Soucek O, Matsykova J, Anliker E, Toigo M, Hlavka Z, Lebl J et al. The muscle-bone interaction in Turner syndrome. Bone 2015; 74: 160–165.
Felson DT, Zhang Y, Hannan MT, Anderson JJ . Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 1993; 8: 567–573.
Reid IR, Plank LD, Evans MC . Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab 1992; 75: 779–782.
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW . Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007; 92: 1640–1646.
Rosen CJ, Bouxsein ML . Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2006; 2: 35–43.
Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res 1999; 14: 2067–2078.
Mohamed-Ali V, Pinkney JH, Coppack SW . Adipose tissue as an endocrine and paracrine organ. In J Obes Rel Metab Disord 1998; 22: 1145–1158.
Ding C, Parameswaran V, Udayan R, Burgess J, Jones G . Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab 2008; 93: 1952–1958.
Anliker E, Toigo M . Functional assessment of the muscle bone unit in the lower leg. J Musculoskelet Neuronal Interact 2012; 12: 46–55.
Erhardt J, Estes J, Pfeiffer C, Biesalski H, Craft N . Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004; 134: 3127–3132.
Mazess RB, Cameron JR . Skeletal growth in school children: maturation and bone mass. Am J Phys Anthropol 1971; 35: 399–407.
Schoenau E . The peak bone mass concept: is it still relevant? Pediatr Nephrol 2004; 19: 825–831.
Judex S, Carlson KJ . Is bone’s response to mechanical signals dominated by gravitational loading? Med Sci Sports Exerc 2009; 41: 2037–2043.
Kohrt WM, Barry DW, Schwartz RS . Muscle forces of gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc 2009; 41: 2050–2055.
Rauch F, Schoenau E . The developing bone: slave or master of its cells and molecules? Pediatr Res 2001; 50: 309–314.
Binkley TL, Specker BL, Wittig TA . Centile curves for bone densitometry measurements in healthy males and females ages 5-22 yr. J Clin Desitom 2002; 5: 343–353.
Zanchetta JR, Plotkin H, Alvarez Filgueira ML . Bone mass in children: normative values for the 2-20-year-old population. Bone 1995; 16 (4 Suppl): 393S–399S.
Teitelbaum SL . Bone resorption by osteoclasts. Science 2000; 289: 1504–1508.
Bacon L, Stern JS, Keim NL, Van Loan MD . Low bone mass in premenopausal chronic dieting obese women. Eur J Clin Nutr 2004; 58: 966–971.
De Pablo P, Cooper MS, Buckley CD . Association between bone mineral density and c-reactive protein in a large population-based sample. Arthritis Rheum 2012; 64: 2624–2631.
Koh JM, Kang YH, Jung CH, Bae S, Kim DJ, Chung YE et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporosis Int 2005; 16: 1263–1271.
Sponholtz TR, Zhang X, Fontes JD, Meigs JB, Cupples LA, Kiel DP et al. Association between inflammatory biomarkers and bone mineral density in a community-based cohort of men and women. Arthritis Care Res 2014; 66: 1233–1240.
Acknowledgements
We kindly thank Dr Marilyn Immoos for reviewing our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mueller, S., Herter-Aeberli, I., Cepeda-Lopez, A. et al. The effect of body composition and serum inflammatory markers on the functional muscle–bone unit in premenopausal women. Int J Obes 41, 1203–1206 (2017). https://doi.org/10.1038/ijo.2017.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.100
This article is cited by
-
The association between dietary inflammatory index, muscle strength, muscle endurance, and body composition in Iranian adults
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Interaction of body fat percentage and height with appendicular functional muscle-bone unit
Archives of Osteoporosis (2019)
-
Are there effects of age, gender, height, and body fat on the functional muscle-bone unit in children and adults?
Osteoporosis International (2018)