Abstract
Background/Objectives:
Uroguanylin and guanylin are secreted by intestinal epithelial cells as prohormones postprandially and act on the hypothalamus to induce satiety. The impact of obesity and obesity-associated type 2 diabetes (T2D) on proguanylin and prouroguanylin expression/secretion as well as the potential role of guanylin and uroguanylin in the control of lipolysis in humans was evaluated.
Subjects/Methods:
Circulating and gastrointestinal expression of proguanylin (GUCA2A) and prouroguanylin (GUCA2B) were measured in 134 subjects. In addition, plasma proguanylin and prouroguanylin were measured before and after weight loss achieved either by Roux-en-Y gastric bypass (RYGB) (n=24) or after a conventional diet (n=15). The effect of guanylin and uroguanylin (1–100 nmol l−1) on lipolysis was determined in vitro in omental adipocytes.
Results:
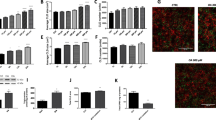
Circulating concentrations of prouroguanylin, but not proguanylin, were decreased in obesity in relation to adiposity. Weight loss achieved by RYGB increased plasma proguanylin and prouroguanylin. Obese T2D individuals showed higher expression of intestinal GUCA2A as well as of the receptors of the guanylin system, GUCY2C and GUCY2D, in omental adipocytes. The incubation with guanylin and uroguanylin significantly stimulated lipolysis in differentiated omental adipocytes, as evidenced by hormone-sensitive lipase phosphorylation at Ser563, an increase in fatty acids and glycerol release together with an upregulation of several lipolysis-related genes, including AQP3, AQP7, FATP1 or CD36.
Conclusions:
Both guanylin and uroguanylin trigger lipolysis in human visceral adipocytes. Given the lipolytic action of the guanylin system on visceral adipocytes, the herein reported decrease of circulating prouroguanylin concentrations in obese patients may have a role in excessive fat accumulation in obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Murphy KG, Bloom SR . Gut hormones and the regulation of energy homeostasis. Nature 2006; 444: 854–859.
Frühbeck G . Gastrointestinal hormones: uroguanylin-a new gut-derived weapon against obesity? Nat Rev Endocrinol 2011; 8: 5–6.
Seeley RJ, Tschöp MH . Uroguanylin: how the gut got another satiety hormone. J Clin Invest 2011; 121: 3384–3386.
Begg DP, Steinbrecher KA, Mul JD, Chambers AP, Kohli R, Haller A et al. Effect of guanylate cyclase-C activity on energy and glucose homeostasis. Diabetes 2014; 63: 3798–3804.
Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR et al. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA 2007; 104: 14507–14512.
Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH et al. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci USA 1993; 90: 10464–10468.
Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL et al. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA 1992; 89: 947–951.
Folgueira C, Sánchez-Rebordelo E, Barja-Fernández S, Leis R, Tovar S, Casanueva FF et al. Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur J Nutr 2016; 55: 529–536.
Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P et al. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol 2010; 20: 1438–1444.
Arakawa H, Kelliher KR, Zufall F, Munger SD . The receptor guanylyl cyclase type D (GC-D) ligand uroguanylin promotes the acquisition of food preferences in mice. Chem Senses 2013; 38: 391–397.
Folgueira C, Beiroa D, Callon A, Al-Massadi O, Barja-Fernández S, Senra A et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes 2016; 65: 421–432.
Frühbeck G, Gómez-Ambrosi J . Rationale for the existence of additional adipostatic hormones. FASEB J 2001; 15: 1996–2006.
Frühbeck G, Gómez-Ambrosi J, Salvador J . Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J 2001; 15: 333–340.
Rodríguez A, Gómez-Ambrosi J, Catalán V, Gil MJ, Becerril S, Sáinz N et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009; 33: 541–552.
American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015; 38 (Suppl): S8–S16.
Catalán V, Gómez-Ambrosi J, Rotellar F, Silva C, Rodríguez A, Salvador J et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res 2007; 39: 495–500.
Muruzabal FJ, Frühbeck G, Gómez-Ambrosi J, Archanco M, Burrell MA . Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen Comp Endocrinol 2002; 128: 149–152.
Xu X, De Pergola G, Eriksson PS, Fu L, Carlsson B, Yang S et al. Postreceptor events involved in the up-regulation of beta-adrenergic receptor mediated lipolysis by testosterone in rat white adipocytes. Endocrinology 1993; 132: 1651–1657.
Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem 2003; 278: 48617–48626.
Rodríguez A, Catalán V, Gómez-Ambrosi J, García-Navarro S, Rotellar F, Valentí V et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab 2011; 96: E586–E597.
Simões-Silva L, Moreira-Rodrigues M, Quelhas-Santos J, Fernandes-Cerqueira C, Pestana M, Soares-Silva I et al. Intestinal and renal guanylin peptides system in hypertensive obese mice. Exp Biol Med (Maywood) 2013; 238: 90–97.
Arner P . Catecholamine-induced lipolysis in obesity. Int J Obes Relat Metab Disord 1999; 23 (Suppl 1): 10–13.
Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J . Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J 2000; 14: 1345–1351.
Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 2000; 97: 787–792.
Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest 2011; 121: 3578–3588.
Frühbeck G, Díez Caballero A, Gil MJ . Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med 2004; 350: 308–309.
Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB . Peptide YY levels are elevated after gastric bypass surgery. Obesity 2006; 14: 194–198.
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Frühbeck G . Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol 2015; 11: 465–477.
Kim GW, Lin JE, Waldman SA . GUCY2C: at the intersection of obesity and cancer. Trends Endocrinol Metab 2013; 24: 165–173.
Akieda-Asai S, Sugiyama M, Miyazawa T, Koda S, Okano I, Senba K et al. Involvement of guanylin and GC-C in rat mesenteric macrophages in resistance to a high-fat diet. J Lipid Res 2013; 54: 85–96.
Mollace V, Salvemini D, Anggard E, Vane J . Nitric oxide from vascular smooth muscle cells: regulation of platelet reactivity and smooth muscle cell guanylate cyclase. Br J Pharmacol 1991; 104: 633–638.
Frühbeck G . Obesity: aquaporin enters the picture. Nature 2005; 438: 436–437.
Yang Y, Chen M, Loux TJ, Harmon CM . Regulation of FAT/CD36 mRNA gene expression by long chain fatty acids in the differentiated 3T3-L1 cells. Pediatr Surg Int 2007; 23: 675–683.
Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X . CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J 2012; 26: 4733–4742.
Richards MR, Harp JD, Ory DS, Schaffer JE . Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res 2006; 47: 665–672.
Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 2006; 55: 3229–3237.
Kishida K, Shimomura I, Kondo H, Kuriyama H, Makino Y, Nishizawa H et al. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J Biol Chem 2001; 276: 36251–36260.
Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P . Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999; 402: 184–187.
Rodríguez A, Moreno NR, Balaguer I, Méndez-Gimenez L, Becerril S, Catalán V et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci Rep 2015; 5: 12067.
Acknowledgements
We thank Beatriz Ramírez (Metabolic Research Laboratory, Clínica Universidad de Navarra) for technical assistance. This work was supported by Fondo de Investigación Sanitaria-FEDER (PI12/00515 and PI13/01430) from the Spanish Instituto de Salud Carlos III, the Department of Health of the Gobierno de Navarra (61/2014) and by the Plan de Investigación de la Universidad de Navarra (PIUNA 2011-14). CIBER de Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of the Instituto de Salud Carlos III, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Rodríguez, A., Gómez-Ambrosi, J., Catalán, V. et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int J Obes 40, 1405–1415 (2016). https://doi.org/10.1038/ijo.2016.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.66
This article is cited by
-
Construction of circRNA-related ceRNA networks in longissimus dorsi muscle of Queshan Black and Large White pigs
Molecular Genetics and Genomics (2022)
-
Activation of brown adipose tissue in diet-induced thermogenesis is GC-C dependent
Pflügers Archiv - European Journal of Physiology (2020)
-
Two distinct GUCY2C circuits with PMV (hypothalamic) and SN/VTA (midbrain) origin
Brain Structure and Function (2019)
-
Circulating Pro-Uroguanylin Levels In Children And Their Relation To Obesity, Sex And Puberty
Scientific Reports (2018)