Abstract
Background:
There is a growinge body of evidence pointing towards an important role for Toll-like receptors (TLR) especially TLR4 in obesity and metabolic syndrome.
Objective:
Owing to the paucity of data on the effect of the accessory proteins, lipopolysaccharide (LPS)-binding protein (LBP) and soluble CD14 (sCD14) on TLR4 activation, the present study was undertaken to examine the effect of sCD14 and LBP on TLR4 activation in pivotal cells of meta-inflammation, monocytes and adipocytes.
Methods:
The dose–response effects of sCD14 and LBP on TLR4 protein abundance in monocytes obtained from normal human volunteers was determined by flow cytometry and in human-differentiated adipocytes by western blotting. Additionally, the nuclear factor-kappaB (NF-κB) p65 and downstream biomediators interleukin (IL)-1β, IL-8, IL-6 and tumor necrosis factor (TNF)-α were measured in the cell culture supernatants by ELISA (enzyme-linked immunosorbent assay).
Results:
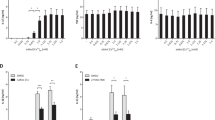
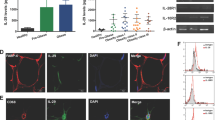
In LPS-primed monocytes, sCD14 but not LBP, augments both TLR4 abundance and inflammatory biomediators (IL-1β, IL-8, IL-6 and TNF-α).sCD14 also showed a similar effect in LPS-primed human adipocytes by augmenting TLR4 protein expression and activity in terms of NF-κB p65 and downstream biomediators (IL-1β, IL-8, IL-6 and TNF-α). LBP at the highest concentration only promoted secretion of IL-8 and TNF-α. However in both monocytes and adipocytes, the effect of sCD14 was superior to LBP.
Conclusions:
In the present report, we make the novel observation that sCD14 compared with LBP, offers a preferred target to ameliorate TLR especially TLR4-induced inflammation and insulin resistance in human obesity and metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fresno M, Alvarez R, Cuesta N . Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem 2011; 3: 151–164.
Watanabe Y, Nagai Y, Tatkatsu K . Activation and regulation of pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients 2013; 5: 3757–3778.
Jialal I, Kaur H, Devaraj S . Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab 2014; 1: 39–48.
Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I . High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008; 57: 3090–3098.
Kitchens RL, Thompson PA . Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res 2005; 11: 225–229.
Lee CC, Avalos AM, Ploegh HL . Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol 2012; 3: 168–179.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772.
Jialal I, Rajamani U . Endotoxemia of metabolic syndrome: a pivotal mediator of meta-inflammation. Metab Syndr Relat Disord 2014; 9: 454–456.
Jialal I, Huet BA, Kaur H, Chien A, Devaraj S . Increased Toll-like receptor activity in patients with metabolic syndrome. Diabetes Care 2012; 35: 900–904.
Devaraj S, Dasu RM, Rockwood J, Winter W, Griffen SC, Jialal I . Increased Toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008; 2: 578–583.
Wang QA, Scherer PE, Gupta RK . Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J Lipid Res 2014; 55: 605–624.
Jialal I, Rajamani U, Adams-Huet B, Kaur H . Circulating pathogen associated molecular pattern - binding proteins and High Mobility Group Box protein 1 in nascent metabolic syndrome: implications for cellular Toll-like receptor activity. Atherosclerosis 2014; 1: 182–187.
Jialal I, Devaraj S, Bettaieb A, Haj F, Adams-Huet B . Increased adipose tissue secretion of Fetuin-A, lipopolysaccharide-bindingprotein and high mobility group box protein 1 in metabolic syndrome. Atherosclerosis 2015; 1: 130–137.
Hardy OT, Kim A, Ciccarelli C, Hayman LL, Wiecha J . Increased Toll-Like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes 2013; 8: e19–e23.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS . TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025.
Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA et al. Loss-of function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 8: 1986–1998.
Pan Z, Zhou L, Hetherington CJ, Zhang DE . Hepatocytes contribute to soluble CD14 production, and CD14 expression is differentially regulated in hepatocytes and monocytes. J Biol Chem 2000; 275: 36430–36435.
Johnson GB, Riggs BL, Platt JL . A genetic basis for the “Adonis” phenotype of low adiposity and strong bones. FASEB J 2004; 18: 1282–1284.
Fernandez-Real JM, Perez del Pulgar S, Luche E, Moreno-Navarrete JM, Waget A, Serino M et al. CD14 modulates inflammation-driven insulin resistance. Diabetes 2011; 60: 2179–2186.
Roncon-Albuquerque Jr R, Moreira-Rodrigues M, Faria B, Ferreira AP, Cerqueira C, Lourenço AP et al. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci 2008; 83: 502–510.
Su GL, Dorko K, Strom SC, Nussler AK, Wang SC . CD14 expression and production by human hepatocytes. J Hepatol 1999; 31: 435–442.
Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS . Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 1993; 90: 2744–2748.
Read MA, Cordle SR, Veach RA, Carlisle CD, Hawiger J . Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharidein human endothelial cells. Proc Natl Acad Sci USA 1993; 90: 9887–9891.
Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013; 33: 158–164.
Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F . Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One 2013; 8: e54600.
Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012; 36: 1442–1449.
Moreno-Navarrete JM, Escoté X, Ortega F, Serino M, Campbell M, Michalski MC et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia 2013; 11: 2524–2537.
Sun L, Yu Z, Ye X, Zou S, Li H, Yu D et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 2010; 33: 1925–1932.
Lepper PM, Schumann C, Triantafilou K, Rasche FM, Schuster T, Frank H et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol 2007; 1: 25–31.
Weiss J . Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans 2003; 31: 785–790.
Schumann RR . Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans 2011; 39: 989–993.
Moreno-Navarrete JM, Escoté X, Ortega F, Camps M, Ricart W, Zorzano A et al. Lipopolysaccharide binding protein is an adipokine involved in the resilience of the mouse adipocyte to inflammation. Diabetologia 2015; 10: 2424–2434.
Acknowledgements
The study was supported by grant from American Diabetes Association (to IJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Pahwa, R., Devaraj, S. & Jialal, I. The effect of the accessory proteins, soluble CD14 and lipopolysaccharide-binding protein on Toll-like receptor 4 activity in human monocytes and adipocytes. Int J Obes 40, 907–911 (2016). https://doi.org/10.1038/ijo.2016.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.32
This article is cited by
-
Activation of toll-like receptor signaling in endothelial progenitor cells dictates angiogenic potential: from hypothesis to actual state
Cell and Tissue Research (2021)
-
Toll-like receptor bioactivity in endothelial progenitor cells
Cell and Tissue Research (2020)