Abstract
Objectives:
Calorie-restriction during gestation in rats has been seen to produce lasting detrimental effects in the offspring, affecting energy balance control and other related metabolic functions. Our aim was to assess whether leptin supplementation throughout lactation may prevent the dysmetabolic phenotype in adulthood associated with gestational calorie restriction.
Methods:
Three groups of male Wistar rats were followed: the offspring of ad libitum fed dams (controls); the offspring of 20% calorie-restricted dams during gestation (CR); and CR rats supplemented with physiological doses of leptin throughout lactation (CR-Leptin). Pups were weaned with a standard diet (SD) until 4 months of age, and then half of the animals of each group were moved to a Western diet (WD) until 6 months of age. Body weight and food intake were recorded. Energy expenditure, locomotive activity, blood parameters, liver triglycerides (TG), and gene expression and specific proteins in liver and white adipose tissue (WAT) were measured in adulthood.
Results:
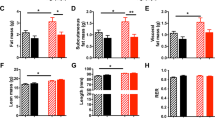
Adult CR rats, but not CR-Leptin rats, displayed greater adiposity index and feed efficiency (both under SD) than controls, along with lower locomotive activity and energy expenditure, higher HOMA-IR index and greater circulating TG and leptin levels. CR animals also exhibited increased values of the respiratory exchange ratio and more severe signs of hepatic steatosis under WD than CR-Leptin animals. Gene expression analysis revealed that CR, but not CR-Leptin, animals displayed indicators of lower capacity for WAT expansion, along with decreased lipogenesis and lipolytic capacity under SD, and impaired lipogenic response of the liver to WD feeding, in accordance with diminished insulin sensitivity and WAT leptin signaling.
Conclusions:
Oral leptin supplementation in physiological doses throughout lactation in rats prevents most of the detrimental effects on energy homeostasis and metabolic alterations in adulthood caused by inadequate fetal nutrition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
WHO. Obesity and overweight. Fact sheet N°3112015, http://www.who.int/mediacentre/factsheets/fs311/en/#.
Malik VS, Willett WC, Hu FB . Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013; 9: 13–27.
Chan RS, Woo J . Prevention of overweight and obesity: how effective is the current Public Health approach. Int J Environ Res Public Health 2010; 7: 765–783.
Barker DJ . The fetal and infant origins of disease. Eur J Clin Invest 1995; 25: 457–463.
Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD . Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 2000; 279: E83–E87.
Sullivan EL, Grove KL . Metabolic imprinting in obesity. Forum Nutr 2010; 63: 186–194.
Picó C, Palou M, Priego T, Sánchez J, Palou A . Metabolic programming of obesity by energy restriction during the perinatal period: different outcomes depending on gender and period, type and severity of restriction. Front Physiol 2012; 3: 436.
Palou M, Priego T, Sanchez J, Torrens JM, Palou A, Pico C . Moderate caloric restriction in lactating rats protects offspring against obesity and insulin resistance in later life. Endocrinology 2010; 151: 1030–1041.
Ravelli GP, Stein ZA, Susser MW . Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976; 295: 349–353.
Thompson NM, Norman AM, Donkin SS, Shankar RR, Vickers MH, Miles JL et al. Prenatal and Postnatal Pathways to Obesity: Different Underlying Mechanisms, Different Metabolic Outcomes. Endocrinology 2007; 148: 2345–2354.
Palou M, Priego T, Sanchez J, Palou A, Pico C . Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr Metab (Lond) 2010; 7: 69.
Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M . Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol 2007; 193: 31–37.
Garcia AP, Palou M, Priego T, Sanchez J, Palou A, Pico C . Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and alphaMSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes Metab 2010; 12: 403–413.
Garcia AP, Palou M, Sanchez J, Priego T, Palou A, Pico C . Moderate caloric restriction during gestation in rats alters adipose tissue sympathetic innervation and later adiposity in offspring. PLoS One 2011; 6: e17313.
Palou M, Priego T, Romero M, Szostaczuk N, Konieczna J, Cabrer C et al. Moderate calorie restriction during gestation programs offspring for lower BAT thermogenic capacity driven by thyroid and sympathetic signaling. Int J Obes (Lond) 2015; 39: 339–345.
Garcia AP, Priego T, Palou M, Sanchez J, Palou A, Pico C . Early alterations in plasma ghrelin levels in offspring of calorie-restricted rats during gestation may be linked to lower sympathetic drive to the stomach. Peptides 2013; 39: 59–63.
Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T et al. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes (Lond) 2007; 31: 1199–1209.
Priego T, Sanchez J, Palou A, Pico C . Leptin intake during the suckling period improves the metabolic response of adipose tissue to a high-fat diet. Int J Obes (Lond) 2010; 34: 809–819.
Miralles O, Sanchez J, Palou A, Pico C . A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006; 14: 1371–1377.
Doneray H, Orbak Z, Yildiz L . The relationship between breast milk leptin and neonatal weight gain. Acta Paediatr 2009; 98: 643–647.
Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J . Leptin in maternal serum and breast milk: association with infants' body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res 2011; 70: 633–637.
Konieczna J, Garcia AP, Sanchez J, Palou M, Palou A, Pico C . Oral leptin treatment in suckling rats ameliorates detrimental effects in hypothalamic structure and function caused by maternal caloric restriction during gestation. PLoS One 2013; 8: e81906.
Konieczna J, Palou M, Sanchez J, Pico C, Palou A . Leptin intake in suckling rats restores altered T3 levels and markers of adipose tissue sympathetic drive and function caused by gestational calorie restriction. Int J Obes (Lond) 2015; 39: 959–966.
Konieczna J, Sanchez J, Palou M, Pico C, Palou A . Blood cell transcriptomic-based early biomarkers of adverse programming effects of gestational calorie restriction and their reversibility by leptin supplementation. Scientific Reports 2015; 5: 9088.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR . Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467–2474.
Diaz-Rua R, Garcia-Ruiz E, Caimari A, Palou A, Oliver P . Sustained exposure to diets with an unbalanced macronutrient proportion alters key genes involved in energy homeostasis and obesity-related metabolic parameters in rats. Food Funct 2014; 5: 3117–3131.
Palou M, Sanchez J, Priego T, Rodriguez AM, Pico C, Palou A . Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. J Nutr Biochem 2010; 21: 23–33.
Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH et al. Removal of Visceral Fat Prevents Insulin Resistance and Glucose Intolerance of Aging: An Adipokine-Mediated Process. Diabetes 2002; 51: 2951–2958.
Palou M, Konieczna J, Torrens JM, Sanchez J, Priego T, Fernandes ML et al. Impaired insulin and leptin sensitivity in the offspring of moderate caloric-restricted dams during gestation is early programmed. J Nutr Biochem 2012; 23: 1627–1639.
Torrens JM, Orellana-Gavalda JM, Palou M, Sanchez J, Herrero L, Pico C et al. Enhancing hepatic fatty acid oxidation as a strategy for reversing metabolic disorders programmed by maternal undernutrition during gestation. Cell Physiol Biochem 2014; 33: 1498–1515.
Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF . Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 1997; 82: 4270–4273.
Houseknecht KL, McGuire MK, Portocarrero CP, McGuire MA, Beerman K . Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem Biophys Res Commun 1997; 240: 742–747.
O'Connor D, Funanage V, Locke R, Spear M, Leef K . Leptin is not present in infant formulas. J Endocrinol Invest 2003; 26: 490.
Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A . Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005; 146: 2575–2582.
Lakshmy R . Metabolic syndrome: role of maternal undernutrition and fetal programming. Rev Endocr Metab Disord 2013; 14: 229–240.
Postic C, Girard J . Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 2008; 118: 829–838.
Choi K, Kim YB . Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med 2010; 25: 119–129.
Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 2012; 18: 388–395.
Mackenzie RW, Elliott BT . Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes 2014; 7: 55–64.
Koschinsky T, Gries FA, Herberg L . Regulation of glycerol kinase by insulin in isolated fat cells and liver of Bar Harbor obese mice. Diabetologia 1971; 7: 316–322.
Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP . Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J 2008; 22: 3925–3937.
Voigt A, Agnew K, van Schothorst EM, Keijer J, Klaus S . Short-term, high fat feeding-induced changes in white adipose tissue gene expression are highly predictive for long-term changes. Mol Nutr Food Res 2013; 57: 1423–1434.
Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ . Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem 2003; 278: 45638–45650.
Huynh FK, Levi J, Denroche HC, Gray SL, Voshol PJ, Neumann UH et al. Disruption of Hepatic Leptin Signaling Protects Mice From Age- and Diet-Related Glucose Intolerance. Diabetes 2010; 59: 3032–3040.
Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD . A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology 2013; 57: 543–554.
Acknowledgements
We thank Enzo Ceresi and Paula Núñez for technical assistance in immunohistochemistry analysis and Western blot analysis, respectively. The research leading to these results was supported by the Spanish Government (grants AGL2012-33692 and AGL2015-67019-P), the European Union’s Seventh Framework Programme FP7 2007-2013 under grant agreements n. 244995 (BIOCLAIMS Project), and the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición, CIBERobn. The Laboratory of Molecular Biology, Nutrition, and Biotechnology (Nutrigenomics) is a member of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: n. FP6-506360). Nara Szostaczuk has been granted a PhD fellowship entitled ‘beca para la formación de personal investigador’, co-funded by the Regional Government (Conselleria d'Educació, Cultura i Universitats, CAIB) and the European Social Fund (FSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AP and CP are authors of a patent held by the University of the Balearic Islands entitled ‘Use of leptin for the prevention of excess body weight and composition containing leptin’ (WO 2006089987 A1; Priority data: 23 February 2005).
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Szostaczuk, N., Priego, T., Palou, M. et al. Oral leptin supplementation throughout lactation in rats prevents later metabolic alterations caused by gestational calorie restriction. Int J Obes 41, 360–371 (2017). https://doi.org/10.1038/ijo.2016.241
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.241
This article is cited by
-
Leptin as a key regulator of the adipose organ
Reviews in Endocrine and Metabolic Disorders (2022)
-
‘Optimising’ breastfeeding: what can we learn from evolutionary, comparative and anthropological aspects of lactation?
BMC Medicine (2020)
-
Blood cell transcript levels in 5-year-old children as potential markers of breastfeeding effects in those small for gestational age at birth
Journal of Translational Medicine (2019)
-
Maternal obesity during lactation may protect offspring from high fat diet-induced metabolic dysfunction
Nutrition & Diabetes (2018)