Abstract
Background/Objectives:
The association between gluten and body weight is inconsistent. Previously, we showed that a gluten-free diet reduces weight gain without changing food intake in mice fed high-fat diets. In the present study, we investigated the effects of gluten intake on fat metabolism, thermogenesis and energy expenditure in mice fed a standard or high-fat diet.
Methods:
Mice were fed four different experimental diets during 8 weeks: a control-standard diet (CD), a CD added with 4.5% of wheat gluten (CD-G), a high-fat diet (HFD) and a HFD added with 4.5% of wheat gluten (HFD-G). After 8 weeks, the mice received 99mTc-radiolabeled gluten orally to study gluten absorption and biodistribution or they underwent indirect calorimetry. After killing, subcutaneous and brown adipose tissues (SAT and BAT) were collected to assess thermogenesis-related protein expression. Lipid metabolism was studied in adipocyte cultures from the four groups.
Results:
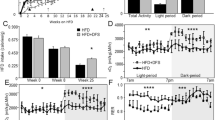
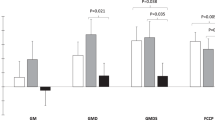
Despite having had the same energy intake, CD-G and HFD-G mice exhibited increased body weight and fat deposits compared with their respective controls. 99mTc-GLU or its peptides were detected in the blood, liver and visceral adipose tissue, suggesting that gluten can even reach extraintestinal organs. Uncoupling protein-1 expression was reduced in the BAT of HFD-G and in the SAT of CD-G and HFD-G mice. Indirect calorimetry showed lower oxygen volume consumption in CD-G and HFD-G groups compared with their controls. In HFD mice, daily energy expenditure was reduced with gluten intake. Gluten also reduced adiponectin, peroxisome proliferator-activated receptor (PPAR)-α and PPARγ and hormone-sensitive lipase in cultures of isolated adipocytes from HFD mice, whereas in the CD-G group, gluten intake increased interleukin-6 expression and tended to increase that of tumor necrosis factor.
Conclusions:
Wheat gluten promotes weight gain in animals on both HFD and CD, partly by reducing the thermogenic capacity of adipose tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wieser H . Chemistry of gluten proteins. Food Microbiol 2007; 24: 115–119.
Shewry PR, Halford NG, Belton PS, Tatham AS . The structure and properties of gluten: an elastic protein from wheat grain. Philos Trans R Soc Lond B Biol Sci 2002; 357: 133–142.
Marcason W . Is there evidence to support the claim that a gluten-free diet should be used for weight loss? J Am Diet Assoc 2011; 111: 1786.
Gaesser GA, Angadi SS . Gluten-free diet: imprudent dietary advice for the general population? J Acad Nutr Diet 2012; 112: 1330–1333.
Soares FL, de Oliveira Matoso R, Teixeira LG, Menezes Z, Pereira SS, Alves AC et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem 2013; 24: 1105–1111.
Faintuch BL, Teodoro R, Duatti A, Muramoto E, Faintuch S, Smith CJ . Radiolabeled bombesin analogs for prostate cancer diagnosis: preclinical studies. Nucl Med Biol 2008; 35: 401–411.
de Barros AL, Mota L, Ferreira CeA, Oliveira MC, Góes AM, Cardoso VN . Bombesin derivative radiolabeled with technetium-99m as agent for tumor identification. Bioorg Med Chem Lett 2010; 20: 6182–6184.
Rodbell M . Metabolism of isolated fat cells. I. effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964; 239: 375–380.
Jönsson T, Ahrén B, Pacini G, Sundler F, Wierup N, Steen S et al. A Paleolithic diet confers higher insulin sensitivity, lower C-reactive protein and lower blood pressure than a cereal-based diet in domestic pigs. Nutr Metab (Lond) 2006; 3: 39.
Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006; 41: 408–419.
Thomas KE, Sapone A, Fasano A, Vogel SN . Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol 2006; 176: 2512–2521.
Fasano A . Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91: 151–175.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993; 366: 740–742.
Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP . Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 1995; 96: 2914–2923.
Klingenberg M, Huang SG . Structure and function of the uncoupling protein from brown adipose tissue. Biochim Biophys Acta 1999; 1415: 271–296.
Bartelt A, Heeren J . Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10: 24–36.
Klingenspor M, Herzig S, Pfeifer A . Brown fat develops a brite future. Obes Facts 2012; 5: 890–896.
Boon MR, van den Berg SA, Wang Y, van den Bossche J, Karkampouna S, Bauwens M et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One 2013; 8: e74083.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004.
Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T et al. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 2005; 4: 147–155.
Lee JY, Hashizaki H, Goto T, Sakamoto T, Takahashi N, Kawada T . Activation of peroxisome proliferator-activated receptor-α enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun 2011; 407: 818–822.
Deng T, Shan S, Li PP, Shen ZF, Lu XP, Cheng J et al. Peroxisome proliferator-activated receptor-gamma transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 2006; 147: 875–884.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001; 50: 2094–2099.
Ouchi N, Parker JL, Lugus JJ, Walsh K . Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97.
Hotamisligil GS, Budavari A, Murray D, Spiegelman BM . Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest 1994; 94: 1543–1549.
Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP . Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 1997; 82: 1313–1316.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
Samaras K, Botelho NK, Chisholm DJ, Lord RV . Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010; 18: 884–889.
Acknowledgements
This study was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), PRPq (Pro-reitoria de Pesquisa of UFMG), FAPEMIG (Fundação de Amparo a Pesquisa de Minas Gerais) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). We thank Maria Helena Alves for animal care and all LABiN (Laboratório de Aterosclerose e Bioquímica Nutricional) members for their contributions and assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Freire, R., Fernandes, L., Silva, R. et al. Wheat gluten intake increases weight gain and adiposity associated with reduced thermogenesis and energy expenditure in an animal model of obesity. Int J Obes 40, 479–486 (2016). https://doi.org/10.1038/ijo.2015.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.204
This article is cited by
-
Gluten worsens non-alcoholic fatty liver disease by affecting lipogenesis and fatty acid oxidation in diet-induced obese apolipoprotein E-deficient mice
Molecular and Cellular Biochemistry (2023)
-
Association of Fructo-oligosaccharides and Arginine Improves Severity of Mucositis and Modulate the Intestinal Microbiota
Probiotics and Antimicrobial Proteins (2023)
-
Physical exercise, obesity, inflammation and neutrophil extracellular traps (NETs): a review with bioinformatics analysis
Molecular Biology Reports (2021)
-
Gluten intake and metabolic health: conflicting findings from the UK Biobank
European Journal of Nutrition (2021)
-
Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes
Sports Medicine (2019)