Abstract
Background/Objectives
Ghrelin is an orexigenic hormone that increases food intake, adiposity, and insulin resistance through its receptor Growth Hormone Secretagogue Receptor (GHS-R). We previously showed that ghrelin/GHS-R signaling has important roles in regulation of energy homeostasis, and global deletion of GHS-R reduces obesity and improves insulin sensitivity by increasing thermogenesis. However, it is unknown whether GHS-R regulates thermogenic activation in adipose tissues directly.
Methods
We generated a novel adipose tissue-specific GHS-R deletion mouse model and characterized the mice under regular diet (RD) and high-fat diet (HFD) feeding. Body composition was measured by Echo MRI. Metabolic profiling was determined by indirect calorimetry. Response to environmental stress was assessed using a TH-8 temperature monitoring system. Insulin sensitivity was evaluated by glucose and insulin tolerance tests. Tissue histology was analyzed by hematoxylin/eosin and immunofluorescent staining. Expression of genes involved in thermogenesis, angiogenesis and fibrosis in adipose tissues were analyzed by real-time PCR.
Results
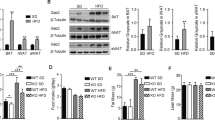
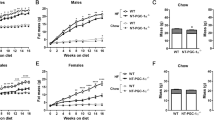
Under RD feeding, adipose tissue-specific GHS-R deletion had little or no impact on metabolic parameters. However, under HFD feeding, adipose tissue-specific GHS-R deletion attenuated diet-induced obesity and insulin resistance, showing elevated physical activity and heat production. In addition, adipose tissue-specific GHS-R deletion increased expression of master adipose transcription regulator of peroxisome proliferator-activated receptor (PPAR) γ1 and adipokines of adiponectin and fibroblast growth factor (FGF) 21; and differentially modulated angiogenesis and fibrosis evident in both gene expression and histological analysis.
Conclusions
These results show that GHS-R has cell-autonomous effects in adipocytes, and suppression of GHS-R in adipose tissues protects against diet-induced obesity and insulin resistance by modulating adipose angiogenesis and fibrosis. These findings suggest adipose GHS-R may constitute a novel therapeutic target for treatment of obesity and metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kursawe R, Caprio S, Giannini C, Narayan D, Lin A, D’Adamo E, et al. Decreased transcription of ChREBP-alpha/beta isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes. 2013;62:837–44.
Lee YH, Mottillo EP, Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta. 2014;1842:358–69.
Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes. 2010;34 Suppl 1:S23–7.
Lin L, Lee JH, Bongmba OY, Ma X, Sheikh-Hamad D, Sun Y. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging. 2014;6:1019–32.
Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–90.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63.
Fang C, Kim H, Noratto G, Sun Y, Talcott ST, Mertens-Talcott SU. Gallotannin derivatives from mango (Mangifera indica L.) suppress adipogenesis and increase thermogenesis in 3T3-L1 adipocytes in part through the AMPK pathway. J Funct Foods. 2018;46:101–9.
Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33.
Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30.
Lee M-J, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371.
Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Investig. 2017;127:74–82.
Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol. 2009;23:914–24.
Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–22.
Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–84.
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48.
Lee JH, Lin L, Wei Q, Bongmba OY, Pradhan G, Sun Y. Neuronal deletion of ghrelin receptor almost completely prevents diet-induced obesity. Diabetes. 2016;65:2169–78.
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61.
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Investig. 2005;115:3564–72.
Lin L, Saha PK, Ma X, Chan L, McGuinness OP, Sun Y. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996–1010.
Wu C-S, Bongmba OY, Yue J, Lee JH, Lin L, Sun Y. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int J Mol Sci. 2017;18:832.
Lin L, Lee JH, Wang R, Sheikh-Hamad D, Zang QS, Sun Y. aP2-Cre mediated ablation of GHS-R attenuates adiposity and improves insulin sensitivity during aging. Int J Mol Sci. 2018;19:3002.
Lin L, Lee JH, Smith CW, Wu H, Sheikh-Hamad D, Sun Y. Ghrelin receptor regulates adipose tissue inflammation in aging. Aging. 2016;8:178–91.
Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94.
Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–31.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76.
Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9:272.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109:5874–9.
Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18:478–89.
Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485.
Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–7.
Lee JH, Eshghjoo S, Davis J, Alaniz RC, Sun Y. New insights on neuronal functions of ghrelin receptor GHS-R in obesity. J Neurol Neuromedicine. 2018;3:69–74.
Edwards A, Abizaid A. Clarifying the ghrelin system’s ability to regulate feeding behaviours despite enigmatic spatial separation of the GHSR and its endogenous ligand. Int J Mol Sci. 2017;18:859.
Rediger A, Piechowski CL, Yi CX, Tarnow P, Strotmann R, Gruters A, et al. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem. 2011;286:39623–31.
Poehlman ET. A review: exercise and its influence on resting energy metabolism in man. Med Sci Sports Exerc. 1989;21:515–25.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Investig. 2011;121:2094–101.
Downes NL, Laham-Karam N, Kaikkonen MU, Yla-Herttuala S. Differential but complementary HIF1alpha and HIF2alpha transcriptional regulation. Mol Ther. 2018;26:1735–45.
Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti-and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–105.
Burr AW, Hillan KJ, McLaughlin KE, Ferrier R, Chapman C, Mathew J, et al. Hepatocyte growth factor levels in liver and serum increase during chemical hepatocarcinogenesis. Hepatology. 1996;24:1282–7.
Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359.
Henry T, Annex B, Azrin M, McKendall G, Willerson J, Hendel R, et al. Double blind, placebo controlled trial of recombinant human vascular endothelial growth factor: the VIVA trial. J Am Coll Cardiol. 1999;33:384A.
Sung H-K, Doh K-O, Son JE, Park JG, Bae Y, Choi S, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72.
Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158:1111–20.
Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83.
Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–12.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9.
Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatry. 2017;22:1056–68.
Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B, et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect. 2020;9:755–68.
Li H, Zhang J, Jia W. Fibroblast growth factor 21: a novel metabolic regulator from pharmacology to physiology. Front Med. 2013;7:25–30.
Acknowledgements
Metabolic analysis was performed in the Mouse Metabolic Research Unit at the USDA/ARS Children’s Nutrition Research Center (CNRC), Baylor College of Medicine. This study was supported by NIH 1R01DK118334 and AG064869, and BrightFocus A2019630S (YS). This work was also supported in part by the USDA National Institute of Food and Agriculture Hatch project 1010840 and Multistate Research NE1939 (YS), NIH P30 ES029067(PI: David Threadgill), and NIH DK109001 (KS).
Author information
Authors and Affiliations
Contributions
JHL, CF, XL, and JYN conducted research and analyzed data; JHL, CF, XY, and YS. wrote the paper. RSC, KS consulted the study. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Fang, C., Li, X. et al. GHS-R suppression in adipose tissues protects against obesity and insulin resistance by regulating adipose angiogenesis and fibrosis. Int J Obes 45, 1565–1575 (2021). https://doi.org/10.1038/s41366-021-00820-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00820-7