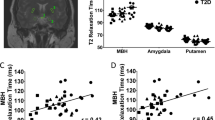
Abstract
Hyperphagia and obesity have been reported following damage to the hypothalamus in humans. Other brain sites are also postulated to be involved in the control of food intake and body weight regulation, such as the amygdala and brainstem. The brainstem, however, is thought to primarily integrate short-term meal-related signals but not affect long-term alterations in body weight, which is controlled by higher centers. The objective of this study was to identify structural pathways damaged in a patient with a brainstem cavernoma who experienced sudden onset of hyperphagia and >50 kg weight gain in <1 year following surgical drainage via a midline suboccipital craniotomy. Diffusion tensor imaging revealed loss of nerve fiber connections between her brainstem, hypothalamus and higher brain centers with preservation of motor tracks. Imaging and endocrine testing confirmed normal hypothalamic structure and function. Gastric bypass surgery restored normal appetite and body weight to baseline. This is the first report of ‘brainstem obesity’ and adds to the brain regions that can determine the long-term body weight set point in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW Central nervous system control of food intake and body weight. Nature 2006; 443: 289–295.
Bray GA, Gallagher TF Jr . Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a reveiw of the literature. Medicine (Baltimore) 1975; 54: 301–330.
King BM Amygdaloid lesion-induced obesity: relation to sexual behavior, olfaction, and the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1201–R1214.
Wren AM, Bloom SR Gut hormones and appetite control. Gastroenterology 2007; 132: 2116–2130.
Grill HJ, Kaplan JM Interoceptive and integrative contributions of forebrain and brainstem to energy balance control. Int J Obes Relat Metab Disord 2001; 25: S73–S77.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004; 23: S208–S219.
MedINRIA: medical image navigation and research tool by INRIA Toussaint N, MedINRIA: medical image navigation and research tool by INRIA Souplet JC, MedINRIA: medical image navigation and research tool by INRIA Fillard P . MedINRIA: medical image navigation and research tool by INRIA In: Proc of MICCAI'07 Workshop on Interaction in Medical Image Analysis and Visualization. Asclepios: Brisbane, Australia, 2007.
Dicks D, Myers RE, Kling A Uncus and amygdala lesions: effects on social behavior in the free-ranging rhesus monkey. Science 1968; 165: 69–71.
Terzian H, Ore GD Syndrome of Kluver and Bucy; reproduced in man by bilateral removal of the temporal lobes. Neurology 1955; 5: 373–380.
Netter FH . Ciba Pharmaceutical Products inc., CIBA-GEIGY Corporation. The Ciba Collection of Medical Illustrations: a Compilation of Pathological and Anatomical Paintings. Ciba Pharmaceutical Products: Summit: NJ, USA, 1959.
Ritter S, Dinh TT, Zhang Y Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 2000; 856: 37–47.
Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 1981; 213: 1036–1037.
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337–345.
le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 2005; 90: 4521–4524.
Boyle CN, Lutz TA Amylinergic control of food intake in lean and obese rodents. Physiology & behavior 2011; 105: 129–137.
Netter FH . Ciba Pharmaceutical Products inc., CIBA-GEIGY Corporation. The Ciba Collection of Medical IIlustrations: a Compilation of Pathological and Anatomical Paintings vol. 1. Ciba Pharmaceutical Products: Summit: NJ, USA, 1959.
Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C Diffusion tensor imaging and white matter tractography in patients with brainstem lesions. Acta Neurochir (Wien) 2007; 149: 1117–1131.
Grill HJ Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity 2006; 14: 216S–221S.
Ahlskog JE, Hoebel BG Overeating and obesity from damage to a noradrenergic system in the brain. Science 1973; 182: 166–169.
Ahlskog JE, Randall PK, Hoebel BG Hupothalamic hyperphagia: dissociation from hyperphagia following destruction of noradrenergic neurons. Science 1975; 190: 399–401.
Wu Q, Clark MS, Palmiter RD Deciphering a neuronal circuit that mediates appetite. Nature 2012; 483: 594–597.
Carter ME, Soden ME, Zweifel LS, Palmiter RD Genetic identification of a neural circuit that suppresses appetite. Nature 2013; 503: 111–114.
Naidich TP, Duvernoy HM Duvernoy's atlas of the human brain stem and cerebellum: high-field MRI: surface anatomy, internal structure, vascularization and 3D sectional anatomy. Springer: Wien; NY, USA, 2009.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630.
Olivan B, Teixeira J, Bose M, Bawa B, Chang T, Summe H et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg 2009; 249: 948–953.
Neary MT, Batterham RL Gut hormones: implications for the treatment of obesity. Pharmacol Ther 2009; 124: 44–56.
Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 2011; 21: 930–934.
Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 2011; 35: 457–461.
Bretault M, Boillot A, Muzard L, Poitou C, Oppert JM, Barsamian C et al. Clinical review: bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J Clin Endocrinol Metab 2013; 98: 2239–2246.
Acknowledgements
We would like to acknowledge the assistance of Drs Madhavi Gaddam and Diana Negreanu with the endocrine workup during their fellowship training period. This work was supported in part by pilot funds through the OHSU Advanced Imaging Research Center and NIH grants R21 DK073729 and R56 DK 88207 (JQP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Purnell, J., Lahna, D., Samuels, M. et al. Loss of pons-to-hypothalamic white matter tracks in brainstem obesity. Int J Obes 38, 1573–1577 (2014). https://doi.org/10.1038/ijo.2014.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.57
Keywords
This article is cited by
-
Brain–gut–microbiome interactions in obesity and food addiction
Nature Reviews Gastroenterology & Hepatology (2020)
-
Neonatal- maternal separation primes zymogenic cells in the rat gastric mucosa through glucocorticoid receptor activity
Scientific Reports (2018)