Abstract
Objective:
Procyanidins are polyphenolic bioactive compounds that exert beneficial effects against obesity and its related diseases. The aim of this study was to evaluate whether supplementation with low doses of a grape seed procyanidin extract (GSPE) to rats during pre- and postnatal periods provides biological effects to their offspring in youth.
Design:
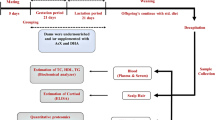
The metabolic programming effect of GSPE was evaluated in the 30-day-old male offspring of four groups of rats that were fed either a standard diet (STD) or a high-fat diet (HFD) and that were supplemented with either GSPE (25 mg kg−1 of body weight per day) or vehicle during pregnancy and lactation.
Results:
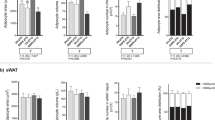
Significant increases in the adiposity index and in the weights of all the white adipose tissue depots studied (retroperitoneal, mesenteric, epididymal (EWAT) and inguinal) were observed in the offspring of rats that were fed a HFD and that were treated with GSPE (HFD-GSPE group) compared with the offspring of rats that were fed the same diet but that did not receive the procyanidins (HFD group). The HFD-GSPE animals also exhibited a higher number of cells in the EWAT, a sharp decrease in the circulating levels of monocyte chemoattractant protein-1 (MCP-1) and a moderate decrease in the plasma glycerol levels. The transcriptomic analysis performed in the EWAT showed 238 genes that were differentially expressed between the HFD and the HFD-GSPE animals, most of which were associated with the immune function and the inflammatory response, in addition to genes associated with adipose tissue remodeling and function, lipid and glucose homeostasis and the metabolism of methyl groups.
Conclusion:
The GSPE treatment in rats that were fed an HFD during pregnancy and lactation induced a clear metabolic programming effect in the offspring, increasing adiposity, decreasing the circulating levels of MCP-1 and changing the gene expression in the EWAT toward a better inflammatory profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A et al. Predicting later-life outcomes of early-life exposures. Environ Health Perspect. 2012; 120: 1353–1361.
Mitra A, Alvers KM, Crump EM, Rowland NE . Effect of high-fat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2009; 296: R20–R28.
Page KC, Malik RE, Ripple Ja, Anday EK . Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1049–R1057.
Bilbo SD, Tsang V . Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 2010; 24: 2104–2115.
Ainge H, Thompson C, Ozanne SE, Rooney KB . A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes 2011; 35: 325–335.
Picó C, Palou M, Priego T, Sánchez J, Palou A . Metabolic programming of obesity by energy restriction during the perinatal period: different outcomes depending on gender and period, type and severity of restriction. Front Physiol 2012; 3: 1–14.
Pan M-H, Lai C-S, Wu J-C, Ho C-T . Epigenetic and disease targets by polyphenols. Curr Pharm Des 2013; 19: 6156–6185.
Arola-Arnal A, Oms-Oliu G, Crescenti A, del Bas JM, Ras MR, Arola L et al. Distribution of grape seed flavanols and their metabolites in pregnant rats and their fetuses. Mol Nutr Food Res 2013; 57: 1741–1752.
Emiliano AF, de Cavalho LCRM, da Silva Cristino Cordeiro V, da Costa CA, de Oliveira PBR, Queiroz EF et al. Metabolic disorders and oxidative stress programming in offspring of rats fed a high-fat diet during lactation: effects of a vinifera grape skin (ACH09) extract. J Cardiovasc Pharmacol 2011; 58: 319–328.
Mukai Y, Sun Y, Sato S . Azuki bean polyphenols intake during lactation upregulate AMPK in male rat offspring exposed to fetal malnutrition. Nutrition 2013; 29: 291–297.
Wu Z, Zhao J, Xu H, Lyv Y, Feng X, Fang Y et al. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur J Nutr 2014; e-pub ahead of print 26 February 2014.
Bladé C, Arola L, Salvadó M-J . Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res 2010; 54: 37–59.
Del Bas JM, Ricketts ML, Baiges I, Quesada H, Ardevol A, Salvadó MJ et al. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol Nutr Food Res 2008; 52: 1172–1181.
Del Bas JM, Ricketts M-L, Vaqué M, Sala E, Quesada H, Ardevol A et al. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol Nutr Food Res 2009; 53: 805–814.
Caimari A, del Bas JM, Crescenti A, Arola L . Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes 2012; 37: 576–583.
Pinent M, Bladé C, Salvadó MJ, Blay M, Pujadas G, Fernández-Larrea J et al. Procyanidin effects on adipocyte-related pathologies. Crit Rev Food Sci Nutr 2006; 46: 543–550.
Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 2009; 20: 210–218.
Terra X, Pallarés V, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G et al. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem 2011; 22: 380–387.
Martinez-Micaelo N, González-Abuín N, Terra X, Richart C, Ardèvol A, Pinent M et al. Omega-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate NF-κB activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem J 2012; 441: 653–663.
Pallarès V, Fernández-Iglesias A, Cedó L, Castell-Auví A, Pinent M, Ardévol A et al. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic Biol Med 2013; 60: 107–114.
Enesco M, Leblond CP . Increase in cell number as a factor in the growth of the organs and tissues of the young male rat. J Embryol Exp Morphol 1962; 10: 530–562.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res 2013; 41: D991–D995.
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009; 25: 1091–1093.
Bindea G, Galon J, Mlecnik B . CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics 2013; 29: 661–663.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Yu R, Kim C-S, Kwon B-S, Kawada T . Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006; 14: 1353–1362.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Buettner R, Schölmerich J, Bollheimer LC . High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007; 15: 798–808.
Adam-Perrot A, Clifton P, Brouns F . Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 2006; 7: 49–58.
Décordé K, Teissèdre P-L, Sutra T, Ventura E, Cristol J-P, Rouanet J-M . Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res 2009; 53: 659–666.
Panee J . Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012; 60: 1–12.
Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS . The major green tea polyphenol metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 2008; 9: 1677–1683.
Van Coillie E, Van Damme J, Opdenakker G . The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev 1999; 10: 61–86.
Kurki E, Shi J, Martonen E, Finckenberg P, Mervaala E . Distinct effects of calorie restriction on adipose tissue cytokine and angiogenesis profiles in obese and lean mice. Nutr Metab (Lond) 2012; 9: 64.
Rouault C, Pellegrinelli V, Schilch R, Cotillard A, Poitou C, Tordjman J et al. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 2013; 154: 1069–1079.
Loughrey BV, McGinty A, Young IS, McCance DR, Powell La . Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low-dose atorvastatin treatment: evidence from a randomized controlled trial. Clin Endocrinol (Oxf) 2013; 79: 800–806.
Bousquet M, St-Amour I, Vandal M, Julien P, Cicchetti F, Calon F . High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol Dis 2012; 45: 529–538.
Quinkler M, Bujalska IJ, Tomlinson JW, Smith DM, Stewart PM . Depot-specific prostaglandin synthesis in human adipose tissue: a novel possible mechanism of adipogenesis. Gene 2006; 380: 137–143.
Iyer A, Lim J, Poudyal H, Reid RC, Suen JY, Webster J et al. An inhibitor of phospholipase A2 group IIA modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Diabetes 2012; 61: 2320–2329.
Xu Z, Huang G, Gong W, Zhao Y, Zhou P, Zeng Y et al. Activation of farnesoid X receptor increases the expression of cytokine inducible SH2-containing protein in HepG2 cells. J Interferon Cytokine Res 2012; 32: 517–523.
Huang S, Qi D, Liang J, Miao R, Minagawa K, Quinn T et al. The putative tumor suppressor Zc3h12d modulates toll-like receptor signaling in macrophages. Cell Signal 2012; 24: 569–576.
Brown C, Gaspar J, Pettit A, Lee R, Gu X, Wang H et al. ESE-1 is a novel transcriptional mediator of angiopoietin-1 expression in the setting of inflammation. J Biol Chem 2004; 279: 12794–12803.
Lkhagvadorj S, Qu L, Cai W, Couture OP, Barb CR, Hausman GJ et al. Microarray gene expression profiles of fasting induced changes in liver and adipose tissues of pigs expressing the melanocortin-4 receptor D298N variant. Physiol Genomics 2009; 38: 98–111.
Boyle KB, Hadaschik D, Virtue S, Cawthorn WP, Ridley SH, Rahilly SO et al. The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death Differ 2009; 16: 782–789.
Jang MK, Kim CH, Seong JK, Jung MH . ATF3 inhibits adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun 2012; 421: 38–43.
Veum VL, Dankel SN, Gjerde J, Nielsen HJ, Solsvik MH, Haugen C et al. The nuclear receptors NUR77, NURR1 and NOR1 in obesity and during fat loss. Int J Obes (Lond) 2012; 36: 1195–1202.
Reichert B, Yasmeen R, Jeyakumar SM, Yang F, Thomou T, Alder H et al. Concerted action of aldehyde dehydrogenases influences depot-specific fat formation. Mol Endocrinol 2011; 25: 799–809.
Ehrlund A, Mejhert N, Lorente-Cebrián S, Aström G, Dahlman I, Laurencikiene J et al. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab 2013; 98: E503–E508.
Park J-R, Jung J-W, Lee Y-S, Kang K-S . The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells. Cell Prolif 2008; 41: 859–874.
Suganami T, Tanaka M, Ogawa Y . Adipose tissue inflammation and ectopic lipid accumulation. Endocr J 2012; 59: 849–857.
Dankel SN, Fadnes DJ, Stavrum A-K, Stansberg C, Holdhus R, Hoang T et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS One 2010; 5: e11033.
Yu X, Shen N, Zhang M-L, Pan F-Y, Wang C, Jia W-P et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J 2011; 30: 3754–3765.
Bing C, Mracek T, Gao D, Trayhurn P . Zinc-α2-glycoprotein: an adipokine modulator of body fat mass? Int J Obes (Lond) 2010; 34: 1559–1565.
Rodríguez A, Catalán V, Gómez-Ambrosi J, García-Navarro S, Rotellar F, Valentí V et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab 2011; 96: E586–E597.
Westin MaK, Hunt MC, Alexson SEH . Short- and medium-chain carnitine acyltransferases and acyl-CoA thioesterases in mouse provide complementary systems for transport of beta-oxidation products out of peroxisomes. Cell Mol Life Sci 2008; 65: 982–990.
Morton NM, Nelson YB, Michailidou Z, Di Rollo EM, Ramage L, Hadoke PWF et al. A stratified transcriptomics analysis of polygenic fat and lean mouse adipose tissues identifies novel candidate obesity genes. PLoS One 2011; 6: e23944.
Karouzakis E, Gay RE, Gay S, Neidhart M . Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2012; 64: 1809–1817.
Martínez-Uña M, Varela-Rey M, Cano A, Fernández-Ares L, Beraza N, Aurrekoetxea I et al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology 2013; 58: 1296–1305.
Williams KT, Schalinske KL . Tissue-specific alterations of methyl group metabolism with DNA hypermethylation in the Zucker (type 2) diabetic fatty rat. Diabetes Metab Res Rev 2012; 28: 123–131.
Acknowledgements
We gratefully acknowledge the aid of Vanessa Grifoll and Silvia Pijuan, the laboratory technicians. The research described here received funding from ACC1Ó (TECCT11-1-0012) and from the Spanish Ministry of Economy and Competitiveness (MECC), (AGL2010-19585, IMPROBES project).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
del Bas, J., Crescenti, A., Arola-Arnal, A. et al. Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int J Obes 39, 7–15 (2015). https://doi.org/10.1038/ijo.2014.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.159